Usability and Cost-of-Usability of Three Dry-Powder Inhalers (DPIs) in Patients with Chronic Obstructive Pulmonary Disease (COPD): May These Variables Influence the Health Technology Assessment of DPIs?
Roberto W Dal Negro, Massimiliano Povero
DOI10.21767/2572-5548.100012
Roberto W Dal Negro1* and Massimiliano Povero2
1National Centre for Respiratory Pharmacoeconomics & Pharmacoepidemiology, CESFAR, Verona, Italy
2AdRes Health Economics and Outcome Recourses - Torino, Italy
- *Corresponding Author:
- Dal Negro RW
National Centre for Respiratory Pharmacoeconomics & Pharmacoepidemiology
CESFAR, Verona, Italy
Tel: 0039-348-3168888
E-mail: robertodalnegro@gmail.com
Received Date: March 15, 2016; Accepted Date: April 25, 2016; Published Date: April 29, 2016
Citation: Negro RWD, Povero M (2016) Usability and Cost-of-Usability of Three Dry-Powder Inhalers (DPIs) in Patients with Chronic Obstructive Pulmonary Disease (COPD): May These Variables Influence the Health Technology Assessment of DPIs?. Chron Obstruct Pulmon Dis 1:12. doi: 10.21767/2572-5548.100012
Copyright: © 2016 Dal Negro RW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Inhalation devices affect per sé the effectiveness and the outcomes of treatment in chronic obstructive pulmonary disease (COPD), independently of the drug(s) administered. Patients’ usability (PU) of Dry Powder Inhalers (DPIs) further contributes in influencing the adherence to, and the impact of treatment.
Aim: To measure and compare the PU and the PU economic impact of three different DPIs in COPD.
Methods: The PU of Breezhaler, Genuair, Handihaler, and the corresponding cost for achieving a proper actuation were investigated and compared in 158 consecutive COPD out-patients. Patients actuated each device after the nurse’s instruction. The nurse reported all the critical steps occurring during the patient’s actuation; to count the attempts needed for actuating each device properly, and to measure the time required for these procedures.
Results: 70 patients tested the three DPIs (66-68 years; well matched for gender). Mean number of attempts to the first proper actuation was the lowest with Genuair (p < 0.0001). Genuair also proved the easiest to use by the nurse judgement (p < 0.0005); the most easily learned (p < 0.0001), and had a successful rate >40% at the first attempt. The mean training cost/patient was the lowest with Genuair (p < 0.0001). When the training cost for achieving a proper inhalation at the first attempt was added to the drug crude cost, the overall cost was € 51 for Genuair, € 75 for Handihaler, and € 122 for Breezhaler.
Conclusions: When the multifaceted aspects of PU are assessed carefully and objectively, some relevant differences among DPIs become clear. DPIs requiring less actions and a shorter training have a proper usability after less attempts. These DPIs also have the lowest cost-of-usability. Cost-of-usability is a novel cost to add to the crude cost of drug(s) in order to grade effectively the DPIs performances, as it could affect substantially the HTA of some devices.
Keywords
Usability; Handling questionnaire; Breezhaler; Genuair; Handihaler; COPD; Health technology assessment; HTA; Cost
Introduction
Inhalation is the preferred route for assuming respiratory drugs particularly when long-term treatments are needed, as in the case of chronic obstructive pulmonary disease (COPD).
Via the inhalation route, airways are directly targeted; a more rapid onset of action is obtained with smaller doses, and a better efficacy-to-safety ratio is also achieved when compared to systemic treatments [1-4].
In chronic airflow limitation, Patient’s Usability (PU) of inhalation devices is a critical issue because both the effectiveness and the outcomes of whatever treatment can be differently affected, independently of the drug administered.
Actually, this variability can usually depend on several specific aspects attaining the devices, such as: their capability to consent the inhalation of a sufficient respirable fraction of the drug (with a particle size ≤ 6 μ); a good reproducibility of inhaled doses; the stability of doses, and a comfortable usability in daily life, particularly in elder patients [5-9].
Even if the development of the Dry Powder Inhalers (DPIs) represented a substantial progress in the evolution of the inhalation therapy (namely, they do not contain propellants; they optimize the consistency of inhaled drug and the extent of its lung deposition; generally minimize the role of patient’s cooperation and cognition in limiting the effectiveness of inhalation) [10,11], the PU within the family of DPIs can change from device to device, and the effectiveness of treatment can then be influenced accordingly [1,12-14].
Recent studies compared different DPIs and measured analytically all the steps of PU by means of a specific validate questionnaire [9,15-17]: results suggested the hypothesis that PU should be regarded as a multifaceted phenomenon which is able per sé to affect, just starting from its training phase, the impact of treatment also in economic terms [9]. Research Article iMedPub Journals https://www.imedpub.com/ Chronic Obstructive Pulmonary Diseases Vol.1 No.2:12 2016 ©
Aim of the study was to measure and compare the PU and the corresponding cost of three different DPIs in an cohort of COPD patients according to a controlled design based on the objective and careful judgment of an expert nurse supervising the process.
Methods
The operational instrument was the Handling Questionnaire [9,15-17] which was administered (from September to December 2015) to consecutive COPD out-patients needing a long-term inhalation treatment.
Three different DPIs were investigated: the Breezhaler, the Genuair, and the Handihaler. The three devices to compare were different in terms of procedures and number of actions for their actuation (such as 7, 3 and 4 respectively).
The study plan consisted of three steps. In the first step, the functioning of each device was shown carefully to each patient in random order by a professional nurse highly expert in educational programs and specifically trained on the technical and the psychological aspects to the study. In this phase, patients were also required to report their preference in usability and the reason of their choice.
In the second phase of the study (that is, after the nurse had instructed carefully each patient on the functioning of each devices), every patient had to prepare the actuation from each device by him/herself, while the nurse had to check his/her manual technicality.
The nurse had also to report the critical issues encountered by each patient handling each device; to measure the time (in sec.) required for the training procedures with each device; to count the overall number of attempts and the overall time (in sec.) needed for actuating each device properly; the number of attempts and the time (in sec.) for the first proper actuation of each device.
In the third phase of the study, all these data were transformed in economic data for each device. In other words, devices were then investigated and compared in terms of cost for their overall usability track, such as the cost corresponding to time spent by the expert professional nurse for explaining the use of each device properly; for demonstrating exhaustively the manoeuvres for actuation, and to attend and support patients’ up to their first proper actuation with each DPI.
The nurse’s cost per minute was calculated stemming from the mean annual salary of a professional hospital nurse [18] divided by 1512 hours per year (such as 36 h/week for 42 working weeks/yr). The figures obtained were then adjusted to the 2015 value by using figures reported by the Italian Consumer Price Index (CPI) [19]. Breezhaler is a device available in the Italian Market for administering indacaterol, glycopyrronium and the association indacaterol/ glycopyrronium, while Genuair and Handihaler are available only for aclidinium and tipotropium, respectively [20]. A brief description of all corresponding prices is detailed in Table 1.
| Device | Drug | Formulation | Public price |
|---|---|---|---|
| Breezhaler | Indacaterol | inhalation powder 30 cps 150/300 mcg | € 35.75 |
| Glycopyrronium | inhalation powder 30 cps 44 mcg | € 48.26 | |
| Indacaterol/Glycopyrronium | inhalation powder 30 cps 85 mcg + 43 mcg | € 71.5 | |
| Genuair | Aclidinium | 1 fl 60 doses inhalation powder 322 mcg | € 48.26 |
| Handihaler | Tiotropium | 30 cps 18 mcg | € 50.29 |
Table 1: Italian public price for all available formulations of Breezhaler, Genuair and Handihaler.
We defined the cost-of-usability as the sum of the costs due to the nurse’s work for demonstrating each devices, for training the patients specifically, for the careful assessment of all actuation procedures, for consenting the first independent proper actuation to patients, to that of the drug + device, which is an official fixed cost.
Statistics
Descriptive statistics are presented as mean ± standard deviation (SD), or simple percentage, as appropriate.
Possible differences among devices were analyzed using the Welch test, the Wilcoxon test or the ANOVA test, as appropriate. The χ2 test was used for categorical variables.
A p-value <0.05 was accepted as the level of statistical significance for all tests. All analyses were performed using computer software R 3.1.2 [21].
Results
The overall patients’ sample consisted of 158 consecutive COPD out-patients. In particular, 70 patients (44%) tested the three DPIs, while 43 (27%) tested Breezhaler and Genuair, and 45 (28%) Breezhaler and Handihaler.
In baseline, patients in the three device sub-groups were well matched in terms of mean age (ranging 66-68 years), gender, and previous experience with and instruction to DPIs (all p = ns) (Table 2).
| Patient characteristics | Breezhaler | Genuair | Handihaler | p-value |
|---|---|---|---|---|
| Patients (N) | 158 | 113 | 115 | |
| Age (years) | 67.8±10.8 | 66.2±11.0 | 68.4±10.5 | NS |
| Sex (% male) | 41.10% | 45.10% | 39.10% | NS |
| Previous experience with DPI | 72.20% | 74.30% | 73.00% | NS |
| Previous instruction to use of DPI | 67.10% | 70.80% | 69.60% | NS |
DPI: dry powder inhaler
Table 2: Baseline characteristics of patients.
About 50% of patients who tested all devices preferred the Genuair and perceived this device as the easiest to use, and the nurse’s judgement confirmed their opinion.
When only Breezhaler and Genuair were tested, there was not a clear trend, while, about 80% of patients preferred to not choose any device if Genuair is not included in the comparison (Table 3).
| Questions | Tested devices | Breezhaler | Genuair | Handihaler | No one | p-value |
|---|---|---|---|---|---|---|
| Which device is the most preferable?* | All devices | 0% | 49% | 6% | 46% | <0.0001 |
| Breezhaler, Genuair | 23% | 34% | NA | 42% | <0.0001 | |
| Breezhaler, Handihaler | 5% | NA | 16% | 79.00% | <0.0001 | |
| Does the device present some critical points in its practical use?** | All devices | 50% (93%) | 51% (30%) | 80% (87%) | <0.0005 (<0.0001) | |
| Breezhaler, Genuair | 42% (61%) | 33% (23%) | NA | NS (<0.005) | ||
| Breezhaler, Handihaler | 51% (82%) | NA | 76% (89%) | <0.05 (NS) |
*Mean response to 3 questions posed to patients (which one most like/most arouses your curiosity/seems easier to use?) and one posed to the nurse (which one seems easier to use?) ** According to patient judgement (data in brackets refer to nurse judgement)
Table 3: Presence of any problem found after the use of devices: the patients’ vs the nurse’s judgement.
When compared to the other two DPIs, Genuair proved as the least problematic, either according to the patients’ judgement and to the nurse’s opinion (Table 3).
In general, patients tended to underestimate the difficulties encountered when handling the Breezhaler: actually, approximately 50% of patients who tested Breezhaler found some substantial difficulties (both considering all patients, and the subgroup analysis), while this proportion increased up to more than 90% according to the nurse’s judgement (Table 3).
There was a perfect agreement between the patients and the nurse in judging the use of Handihaler as quite difficult. The number of attempts required to patients for preparing the first proper inhalation represented a very important indicator of efficiency and practicality of the devices investigated in the present study.
Mean number of attempts before achieving the first proper actuation was lower with Genuair than with Breezhaler and Handihaler (1.7 vs 2.7-2.8, p<0.0001).
Furthermore, 43% of patients learned how to use Genuair properly after the first demonstration, while almost 90% of patients were unable to use the other two devices properly after the first demonstration (Table 4).
| Breezhaler | Genuair | Handihaler | p-value | ||
|---|---|---|---|---|---|
| Device characteristics | |||||
| Manoeuvres (n)* | 7 | 3 | 4 | ||
| Time spent by nurse with patients during training$ (min) | 1st attempt | 2.8±0.1 | 1.3±0.1 | 1.4±0.1 | p <0.0001 |
| 2nd attempt | 4.2±0.2 | 1.8±0.1 | 2.0±0.2 | p <0.0001 | |
| 3rd attempt | 5.0±0.3 | 2.1±0.2 | 2.7±0.2 | p <0.0001 | |
| Usability | |||||
| n. attempts before achieving the first proper inhalation | 2.8±1.0 | 1.7±0.8 | 2.7±1.1 | p <0.0001 | |
| Successful rate at the first attempt (%) | 11.40% | 43.40% | 11.30% | p <0.0001 | |
| Total time required for the first proper inhalation$ (min) | 11.2±4.9 | 2.7±1.5 | 5.4±2.8 | p <0.0001 | |
* Number of operations to prepare the inhalation
$ including patient time and nurse demonstration
Table 4: Characteristics of devices tested and differences in usability.
The time for the nurse’s explanation and the time needed to patients for preparing the inhalation with Genuair and Handihaler proved similar.
Nevertheless, the total time spent in learning how to use the former device properly was significantly lower than that of the latter device (p < 0.0001).
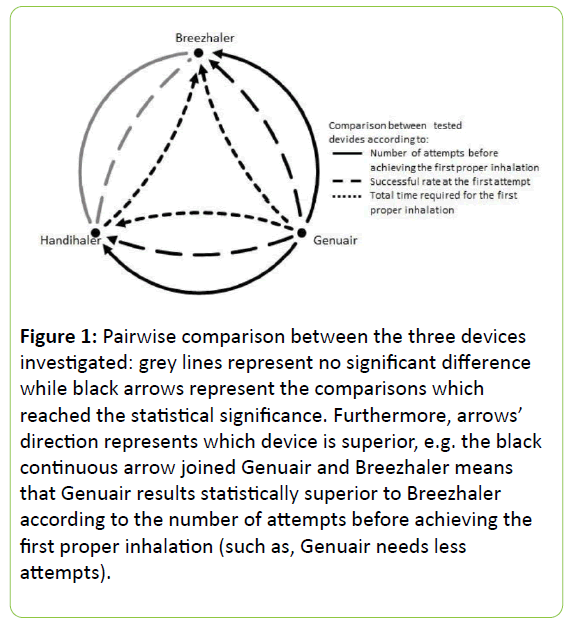
The corresponding total time for Breezhaler was the highest one (about 11 minutes), due to the highest number of manoeuvres and to the longest explanation required (Table 4). The pairwise comparisons among the three devices are reported in Figure 1.
Figure 1: Pairwise comparison between the three devices investigated: grey lines represent no significant difference while black arrows represent the comparisons which reached the statistical significance. Furthermore, arrows’ direction represents which device is superior, e.g. the black continuous arrow joined Genuair and Breezhaler means that Genuair results statistically superior to Breezhaler according to the number of attempts before achieving the first proper inhalation (such as, Genuair needs less attempts).
Genuair resulted the easiest to use and the fastest to be learned (p < 0.0001), while no significant difference was detected between Breezhaler and Handihaler, either in the number of attempts needed to actuate the first proper inhalation, and in the successful rate at the first attempt.
In particular, as Breezhaler required the longest explanation and demonstration of use, the total time needed for the first proper inhalation resulted significantly lower with Handihaler than with Breezhaler (p < 0.0001).
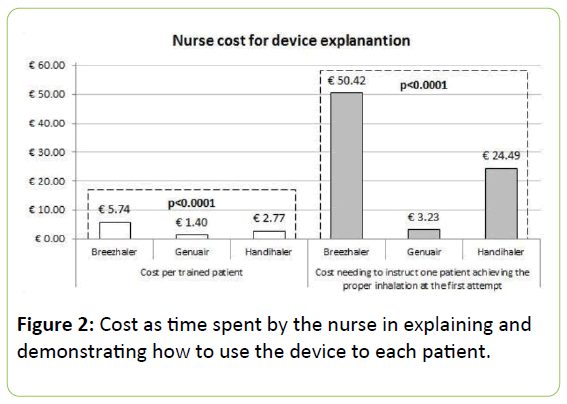
The mean cost related to the training actions (including the nurse’s time for demonstration and that for attending the patients’ manoeuvres) amounted to € 3.59 ± 2.62 per patient (IQR: 1.62 - 6.13).
Genuair proved as the less expensive by a cost per patient of € 1.40 ± 0.76 (IQR: 0.68 - 1.62), corresponding to half and a quarter of the cost estimated for Handihaler and Breezhaler, respectively (Figure 2).
Furthermore, the cost needing to instruct a patient properly (i.e., such that for obtaining at least one patient exhaustively learned on how to use each device correctly at the first attempt) grew exponentially with Breezhaler and Handihaler (up to about € 50 and 25, respectively), while with Genuair it proved lower than € 4 (Figure 2).
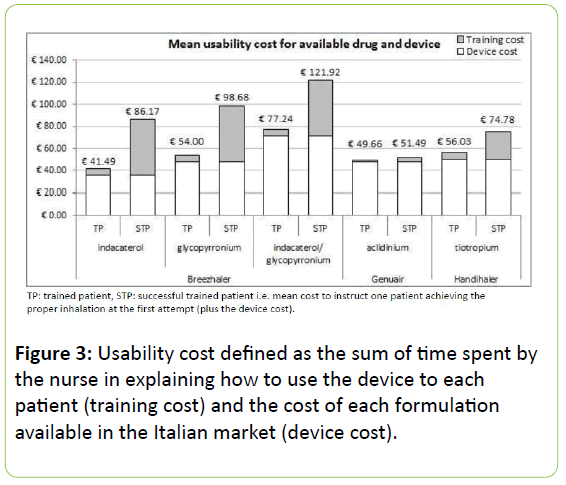
When the cost of each device was included, the cost-of-usability for Breezhaler ranged between € 40-80, according to the administered drug contained. This cost increased up to € 122 if the cost for leading one patient to perform a proper inhalation at the first attempt was considered.
The corresponding cost for Handihaler resulted in € 56 or € 75, according to the training cost considered. No significant differences were instead detected in the cost-of-usability for Genuair, which remained stable around € 50 per patient (Figure 3).
Discussion
Respiratory drugs are preferably delivered via the inhalation route. Nevertheless, the choice of the inhaler device to prescribe is frequently empirically guided in real life, being the technological characteristics and the effective performance of different devices usually underestimated or neglected [22-25]. Moreover, it should be took into account that the skill in the device usability is frequently inadequate also among health care professionals (such as: GPs, medical students, respiratory physiotherapists, pharmacists, and even nurses and lung physicians), [14] who then cannot support their patients conveniently from this point of view. On the contrary, specific education and training are essential in order to maintain and improve the proper usability of prescribed device(s), even though it represents a critical issue indeed in daily activity because it is time consuming, [21] and specific professional figures are frequently insufficient or missing.
Patients’ usability is a multifaceted issue, particularly if devices require a complicated sequence of manoeuvres for their proper actuation, and the procedures are not preceded by or associated to a sufficient instruction and training [4,26].
As COPD patients usually need long-term inhalation treatments and need to be familial with their device(s) for long periods, their PU is crucial point to assess for its possible consequences in terms of therapeutic strategy and effectiveness. Actually, the interest in PU revamped in recent years [25,27-32] due to the increased awareness that PU would contribute to a better adherence to treatment, thus leading to an increased effectiveness of treatment.
Just stemming from this evidence, PU and the assessment of the process for the proper actuation are of great value because they can greatly contribute to differentiate the performances of different DPIs and their impact. Grading the performance of each device in terms of both usability and therapeutic convenience in daily life would represent a strong decisional index, [26,27] particularly when derived from the utilization of specific, comprehensive, objective, and validated investigational tools [15-17,25,28].
Results of the present study showed that substantial differences are existing within devices belonging to the DPIs family, particularly when compared in terms of their PU and corresponding economic impact. Genuair was the device which was characterized by the lowest degree of difficulties in understanding the manoeuvres needed for actuating the inhalation properly and effectively. Moreover, Genuair proved the easiest to use and the least problematic (p < 0.0005), the most easily learned (p < 0.0001), and that one with a successful rate of more than 40% at the first attempt (p < 0.0001). These data are confirming results of some previous studies which documented Genuair as the most preferred device when compared to other DPIs (namely, Breezhaler and Handihaler) [30-33].
To note that there was a substantial discrepancy between the patients’ belief “at glance” (such as, not based on true handling skills, but only on subjective perceptions) and the effective PU with some devices in real lie. It is the peculiar case of Breezhaler which, easy to use “at glance”, confirms this misleading perception together with the conflicting results already reported between the patients’ opinion and the objective judgment of the nurse who is carefully attending all the steps of the patients’ actuation procedures [17].
If the fundamental principle of any therapeutic strategy should be the prescription of the most effective and costeffective agent(s), the choice of the most effective inhalation device should also be valued, because the patient’s usability of the device can affect the adherence, the outcomes, and the cost of treatment even substantially, independently of the drug used [34]. This evidence was emphasized in the present study where Genuair proved the most convenient DPI in COPD patients, because its cost-of-usability was absolutely the lowest when compared to that of the other two DPIs investigated, and the extra-cost spent for instructing and training the patient until the proper inhalation confirmed as the main determinant. Actually, Breezhaler is the only DPI of those investigated which is usable at present for different molecules. When Indacaterol was administered via Breezhaler, it appears as the most convenient, with an apparent cost-perpatient of about € 40 (such as, the cost of the device + that of the drug + that of training), but only 11% of patients were able to handle it properly. In other words, about 10 patients should be trained with Breezhaler to have one patient able to actuate the device properly after the first demonstration. In economic terms, this corresponds to a cost-of-usability per patient of about € 85 for Indacaterol.
Conclusions
DPIs available in the present and in the future market are characterized by several peculiarities concerning their technology and usability which make their convenience different from each other even if belonging to the same family of inhalation devices. Independently of their shape, size and other exterior characteristics, the number of manoeuvres for their actuation; the time for understanding their inhalation procedures; the time for overall training, and the time for proper usability represent the main characteristics which can vary substantially from each other, and may then lead to a different PU and a different convenience, also in economic terms.
PU and convenience are variables which can only be assessed and compared by means of specific and objective measures which contribute to grade the comprehensive performance in real-life of each DPI from this point of view. Namely, the number of patients’ attempts for the first proper actuation, together to the successful rate at the first attempt, and to the total time required for the first proper actuation are the three main determinants of PU which consequently greatly contribute to define the profile of economic convenience of each device. These variables, never previously included into the assessing track of PU to our knowledge, are suggesting their possible substantial impact also in terms of Health Technology Assessment (HTA) of DPIs. Cost-of-usability is a novel cost (never previously used to our knowledge) which should to be considered together to the crude cost of drug because may affect the HTA of these devices even substantially.
When compared to Breezhaler and Handihaler from this point of view, Genuair proved the DPI characterized by the most convenient cost-of-usability.
References
- Cross S (2001) Asthma inhalation delivery systems: the patient's viewpoint. J Aerosol Med 14: 83-87.
- Magnussen H (2005) Novolizer: how does it fit into inhalation therapy? Curr Med Res Opin 21: S39-46.
- Barnes PJ (2005) Introduction: how can we improve asthma management? Curr Med Res Opin 21: S1-3.
- Melani AS (2007) Inhalatory therapy training: a priority challenge for the physician. Acta Biomed 78: 233-245.
- Newman SP, Busse WW (2002) Evolution of dry powder inhaler design, formulation, and performance. Respir Med 96: 293-304.
- Wieshammer S, Dreyhaupt J (2008) Dry powder inhalers: which factors determine the frequency of handling errors? Respiration 75: 18-25.
- Chapman KR, Fogarty CM, Peckitt C, Lassen C, Jadayel D, et al. (2011) Delivery characteristics and patients' handling of two single-dose dry-powder inhalers used in COPD. Int J Chron Obstruct Pulmon Dis 6: 353-363.
- Barrons R, Pegram A, Borries A (2011) Inhaler device selection: special considerations in elderly patients with chronic obstructive pulmonary disease. Am J Health Syst Pharm 68: 1221-1232.
- Dal Negro RW, Povero M (2016) The economic impact of educational training assessed by the Handling Questionnaire with three inhalation devices in asthma and COPD patients. ClinicoEconomics and Outcomes Research, in press.
- Virchow JC (2004) Guidelines versus clinical practice--which therapy and which device? Respir Med 98: S28-34.
- Virchow JC (2005) What plays a role in the choice of inhaler device for asthma therapy? Curr Med Res Opin 21 : S19-25.
- Virchow JC, Crompton GK, Dal Negro R, Pedersen S, Magnan A, et al. (2008) Importance of inhaler devices in the management of airway disease. Respir Med 102: 10-19.
- Lenney J, Innes JA, Crompton GK (2000) Inappropriate inhaler use: assessment of use and patient preference of seven inhalation devices. EDICI. Respir Med 94: 496-500.
- Self TH, Arnold LB, Czosnowski LM, Swanson JM, Swanson H (2007) Inadequate skill of healthcare professionals in using asthma inhalation devices. J Asthma 44: 593-598.
- Dal Negro RW, Guerriero M (2008) Cultural and linguistic testing of the Handling Questionnaire: a specific instrument for assessing the patient's acceptability of dry powder inhalers. Monaldi Arch Chest Dis 69: 170-177.
- PerpiñáTordera M, Viejo JL, Sanchis J, Badia X, Cobos N, et al. (2008) Assessment of patient satisfaction and preferences with inhalers in asthma with the FSI-10 questionnaire. Arch Bronconeumol 44: 346-352.
- Dal Negro RW, Povero M (2016) Acceptability and preference of three inhalation devices assessed by the Handling Questionnaire in asthma and COPD patients. Multidisciplinary Respiratory Medicine 11: 7.
- Favalli EG, Marchesoni A, Colombo GL, Sinigaglia L (2008) Pattern of use, economic burden and vial optimization of infliximab for rheumatoid arthritis in Italy. ClinExpRheumatol 26: 45-51.
- Harmonised Indices of Consumer Prices (HICPs) (2014) European Commission EuroStat.
- List of transparency of medicines included in the list of equivalent drugs ( Law 178/2002 ) with their reference prices , including the reduction in accordance with the determination AIFA July 3, 2006 , the further reduction of 5% for the purposes of determining AIFA of 27 September 2006 and article 9 paragraph 1 of Law 31 of February 28, 2008 ( Pay back ) and the article 11 paragraph 9 of the DL78 / 2010 converted with amendments by the Law of 30 July 2010 , n .122 . Last update December 15 , 2015 .
- Dean CB, Nielsen JD (2007) Generalized linear mixed models: a review and some extensions. Lifetime Data Anal 13: 497-512.
- Barry PW, O'Callaghan C (2003) The influence of inhaler selection on efficacy of asthma therapies. Adv Drug Deliv Rev 55: 879-923.
- Anderson P (2005) Patient preference for and satisfaction with inhaler devices. EurRespir Rev 96: 109-116.
- Schulte M, Osseiran K, Betz R, Wencker M, Brand P, et al. (2008) Handling of and preferences for available dry powder inhaler systems by patients with asthma and COPD. J Aerosol Med Pulm Drug Deliv 21: 321-328.
- van der Palen J, Ginko T, Kroker A, van der Valk P, Goosens M, et al. (2013) Preference, satisfaction and errors with two dry powder inhalers in patients with COPD. Expert Opin Drug Deliv 10: 1023-1031.
- Chrystyn H (2005) Do patients show the same level of adherence with all dry powder inhalers? Int J ClinPractSuppl149: 19-25.
- van Schayck CP, BjilHofiad ID, Folgering H, Cloosterman SG, Akkermans R, et al. (2002) Influence of two different inhalation devices on therapy compliance in asthmatic patients. Scand J Prim Health Care 20: 126-128.
- Anderson P (2006) Use of Respimat Soft Mist inhaler in COPD patients. Int J Chron Obstruct Pulmon Dis 1: 251-259.
- Lavorini F, Fontana GA (2014) Inhaler technique and patient's preference for dry powder inhaler devices. Expert Opin Drug Deliv 11: 1-3.
- Beier J, Kirsten AM, Mróz R, Segarra R, ChuecosF, et al. (2013) Efficacy and safety of aclidinium bromide compared with placebo and tiotropium in patients with moderatetosevere chronic obstructive pulmonary disease: results from a 6week, randomized, controlled Phase IIIb study. COPD 10: 511-522.
- der PalenJv (2014) Genuair(®) in chronic obstructive pulmonary disease: a novel, user-friendly, multidose, dry-powder inhaler. TherDeliv 5: 795-806.
- Chrystyn H, Niederlaender C (2012) The Genuair® inhaler: a novel, multidose dry powder inhaler. Int J ClinPract 66: 309-317.
- Pascual S, Feimer J, De Soyza A, SauledaRoig J, Haughney J, et al. (2015) Preference, satisfaction and critical errors with Genuair and Breezhaler inhalers in patients with COPD: a randomised, cross-over, multicentre study. NPJ Prim Care Respir Med 25: 15018.
- Brocklebank D, Ram F, Wright J, Barry P, Cates C, et al. (2001) Comparison of the effectiveness of inhaler device in asthma and chronic obstructive airways disease: a systematic review of the literature. Health Technol Assess 5: 1-149.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences