Minute lesions of alveolar damages in lungs of patients with stable chronic obstructive pulmonary disease
Iwao Emura, Hiroyuki Usuda and Kazuhiro Satou
DOI10.21767/2572-5548.100028
Iwao Emura1*, Hiroyuki Usuda1 and Kazuhiro Satou2
1Departments of Surgical Pathology, Japanese Red Cross Nagaoka Hospital, Nagaoka City, Japan
2 Departments of Internal Medicine, Japanese Red Cross Nagaoka Hospital, Nagaoka City, Japan
- *Corresponding Author:
- Emura I
Department of Surgical Pathology, Japanese
Red Cross Nagaoka Hospital, Senshyuu
Nagaoka City, Japan
Tel: 0258-28-3600
Fax: 0258-28-9000
E-mail: emura@nagaoka.jrc.or.jp
Received date: November 12, 2017; Accepted date: December 19, 2017; Published date: December 26, 2017
Citation: Emura I, Usuda H, Satou K (2017) Minute Lesions of Alveolar Damages in Lungs of Patients with Stable Chronic Obstructive Pulmonary Disease. Chron Obstruct Pulmon Dis Vol.2 No.2:28 doi: 10.21767/2572-5548.100028
Copyright: © 2017 Emura I. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: To reveal the mechanism underlying recruitment of neutrophils in chronic obstructive pulmonary disease (COPD) lungs and to investigate the role of minute lesions of alveolar damage (MLADs) in emphysema development.
Methods: Seventy-four lobes from 74 patients with stable COPD, and 78 lobes from 78 patients without COPD (controls) were immunohistochemically examined.
Results: MLADs presented as microscopic foci of inflammatory lung injury where alveolar epithelial cells had fragmented and disappeared, and ring-like or tubelike capillary structures had disappeared from the alveolar septae. MLADs were detected in 11 of 74 COPD patients. Tumor necrosis factor (TNF) -α+ macrophages and hypoxia inducible factor (HIF)-2α+ macrophages were detected in 100% of patients in the smoking group with or without COPD and in all patients with COPD exhibiting MLADs. The numbers of neutrophils in alveolar septae and alveolar spaces were significantly larger in the COPD smoking group and non-COPD smoking group than in the non-COPD non-smoking group, and the numbers of neutrophils were tend to be larger in and around MLADs than in lung tissues located away from MLADs in smoking patients with COPD. Masson body-like tissues estimated to be organizations of exudates as well as mild interstitial fibrosis estimated to represent the fibroproliferative phase of MLADs were observed in patients with COPD and smoking patients without COPD.
Conclusion: These findings suggest the followings: (1). HIF-2α+macrophages and TNF-α+macrophages induced by hypoxia caused by smoking play an important role in the recruitment of neutrophils, (2). MLADs develop in lungs in which large number of neutrophils have been recruited probably by deterioration of hypoxia and play an important role in the development of subsequent full-scale emphysema.
Keywords
Chronic obstructive pulmonary disease; Emphysema; Minute lesions of alveolar damage; Tumor necrosis factor-α; Hypoxia inducible factor-2α; Hypoxia; Smoking
Abbreviations
MLADs: Minute lesions of alveolar damage; COPD: chronic obstructive pulmonary disease; TNF-α: Tumor necrosis factor-α; HIF-2α: hypoxia inducible factor-2α
Introduction
Chronic obstructive pulmonary disease (COPD) is a major cause of illness and death worldwide. It affects about 10% of the general population [1], but its prevalence among heavy smokers can reach 50% [2]. The most important cause of COPD in developed countries is cigarette smoking [3,4]. COPD is usually a progressive neutrophilic inflammatory airway disorder that develops after long-term exposure to external stresses, such as tobacco smoke inhalation [5-7]. Recruitment of neutrophils is reportedly mediated by a variety of molecular signals that bind to neutrophil surface receptors; these molecular signals include microbial breakdown products and the chemokine interleukin-8 [8,9]. Neutrophil recruitment may also be induced by release of a potent chemotactic factor for neutrophils by alveolar macrophages [10]. However, we found no reports describing the histopathologic features underlying the neutrophil-associated lung injury in lungs of patients with COPD.
A previous paper reported the existence of minute lesions of alveolar damage (MLADs) in lungs with stable idiopathic pulmonary fibrosis. The formation of MLADs may be initiated by hypoxia; additionally, hypoxia inducible factor-2α-positive (HIF- 2α+) macrophages, tumor necrosis factor-α-positive (TNF-α+) macrophages, and neutrophils were considered to play central roles in the formation of MLADs [11]. We considered that the lungs of smoker are in a hypoxic condition consequence of smoking. Histopathological examination revealed that HIF-2α+ macrophages and TNF-α+ macrophages were present in 100% of smoker with or without COPD and MLADs were present in some patients with stable COPD. These findings suggest that HIF-2α+macrophages and TNF-α+macrophages induced by hypoxia caused by smoking play an important role in the recruitment of neutrophils, and MLADs play an important role in the development of subsequent full-scale emphysema. We herein describe the importance of HIF-2α+ macrophages, TNF-α+ macrophages, and MLADs in the recruitment of neutrophils as well as the histopathologic mechanism underlying neutrophilinduced lung injury in patients with COPDs.
Materials and Methods
Patients
Seventy-four sequential patients with COPD and 78 sequential patients without COPD (controls) obtained by lobectomy for treatments of lung cancer from 2012 to 2016 were retrospectively examined. Spirometry was performed in all patients, and all obtained lobes had the wide architecturally normal lung tissue located apart from the cancer. Patients with complications such as severe heart failure, idiopathic pulmonary fibrosis, tuberculosis and bronchiectasis were excluded from the present study. The Japanese Red Cross Nagaoka Hospital ethics committee approved the study (No. 1189).
Tissue processing and histopathological examination
Lung tissues were fixed in 10% neutral formalin and, embedded in paraffin. Sections were examined after staining with hematoxylin and eosin, Gram stain, Grocott’s variation of methenamine silvernitrate stain, and elastic van Gieson stain.
Immunohistochemical examination
Paraffin sections were examined using Simple Stain MAXPO (Nichrei Co., Tokyo, Japan) with diaminobenzidine as the chromogen using mouse monoclonal anti-human keratin (AE1/ AE3, an epithelial marker, at 1:150: DAKO USA, Carpinteria, CA, USA.), anti-human 3-fucosyl-N-acetyllacetosamine (CD15, a neutrophil marker, at 1:100: Novo Castra, Newcastle upon Tyne), anti-human transmembrane glycoprotein (CD34, an endothelial cell marker, DAKO USA, Carpinteria, CA, USA), antihuman TNF-α (1:100: Abcam, Cambridge, UK), and anti-human HIF-2α (1:100: Abcam). An antigen retrieval method using citrate buffer and microwave heating was employed for all antibodies. As a negative control, the primary antibody was substituted with phosphate-buffered saline, and a positive stain was not observed in these controls.
Clinical diagnosis of COPD
The diagnosis of COPD was made according to established international guidelines [5]. Patients with a post-bronchodilator forced expiratory volume percentage (%) in 1 second of <0.70 were classified into the COPD group. The patients in the COPD and non-COPD groups were further divided into a smoking group (n=29 and 24, respectively), a smoking cessation group (n=35 and 25, respectively), and a non-smoking group (n=10 and 29, respectively).
Histopathological examinations
The diagnosis of MLADs was based on immunohistochemistry with AE1/AE3, and CD34 antibodies. We defined a MLAD as a lung injury characterized by fragmentation and disappearance of alveolar epithelial cells with peri-alveolar cytokeratin-positive cell debris, injury of capillary endothelial cells and mild edema and extravasation. Nodular granulation tissue was defined as small granulation tissues composed of a few shrunken alveoli and surrounding loose fibrosis [11]. Masson body-like tissue was defined as small plaques of loose fibro-myxoid connective tissue within bronchioles and alveoli. We chose areas that were located apart from the cancer and contained the widest architecturally normal lung tissue because MLAD and nodular granulation tissues were found in architecturally normal lung tissue. We examined the presence or absence of MLADs, nodular granulation tissue, Masson body-like tissues and alveolar septal fibrosis in 5 cm2 sections of lung tissue. We also examined the number of CD15- positive neutrophils in air spaces and alveolar septae in 30 highpower fields. In the patients with COPD who exhibited MLADs, we examined the number of CD15-positive neutrophils in air spaces and alveolar septae in and around MLADs and those in lung tissue located away from the MLADs.
Statistical analysis
Continuous variables did not show normal distribution, so, are shown as median (quartiles). Continuous variables were compared using the Kruskal-Wallis test with Scheffe’s method for multiple comparisons and the t-test. Discrete variables were analyzed using the chi-square test and Fisher’s exact test with Ryan’s method for multiple comparisons. A P-value of < 0.05 were considered statistically significant. SPSS statistics 17.0 software (SPSS Japan Inc., Tokyo, Japan) was used for all analyses.
Results
Patient characteristics
The basic characteristics of the patients at surgery are shown in Table 1. No patients with COPD had experienced exacerbation before the operation, and all did not satisfy the diagnostic criteria for exacerbation at the time of surgery.
| COPD | Non-COPD | ||||||
|---|---|---|---|---|---|---|---|
| S. (n=29) | S.C. (n=35) | No S. (n=10) | S. (n=24) | S.C. (n=25) | No S. (n=29) | p | |
| Male | 25(86%) | 35(100%) | 4(40%) | 22(92%) | 21(84) | 2(7%) | <0.001 |
| Age | 66.0(61.0, 73.0) | 71.0(65.0, 78.0) | 77.0 (69.8, 81.0) | 65.0(57.3, 71.0) | 70.0 (65.8, 75.5) | 68.0 (64.0, 74.5) | 0.008 |
| C.I. | 810.0(600.0, 1200.0) | 1100(700.0, 1520.0) | 0.0(0.0, 0.0) | 705.0(412.5, 990.0) | 560.0 (340.0, 1000.0) | 0.0(0.0, 0.0) | <0.001 |
| LABA | 7(24%) | 7(20%) | 1(10%) | 0 | 0 | 0 | <0.201 |
| LAMA | 1(3%) | 3(9%) | 0 | 0 | 0 | 0 | 0.002 |
| LABA+LAMA | 7(24%) | 4(11%) | 0 | 0 | 0 | 0 | 0.001 |
| HIF-2α | 29(100%) | 21(60%) | 2(20%) | 24(100%) | 5(20%) | 1(3%) | <0.001 |
| TNF-α | 29(100%) | 21(60%) | 2(20%) | 24(100%) | 5(20%) | 1(3%) | <0.001 |
| N-s | 128.0(86.5, 160.0) | 90.0(54.0, 156.0) | 50.0 (17.3, 90.8) | 130.0 (109.0, 164.5) | 71.0 (35.0, 119.5) | 54.0(24.5, 100.0) | <0.001 |
| N-a | 42.0(24.5, 71.0) | 32.0(54.0, 63.0) | 7.5(4.8, 12.8) | 36(18.8, 46.8) | 5.0(3.0, 6.0) | 5.0(3.0, 6.0) | <0.001 |
| NGT | 0 | 0 | 0 | 0 | 0 | 0 | - |
| MBLT | 14(48%) | 12(34%) | 1(10%) | 4(17%) | 0 | 0 | <0.001 |
| Fibrosis | 29(97%) | 19(54%) | 4(40%) | 9(38%) | 7(28%) | 1(3%) | <0.001 |
| MLAD | 6(21%) | 3(9%) | 2(20%) | 0 | 0 | 0 | 0.005 |
Table 1: Results in patients with and without COPD.
Pathological findings of MLADs
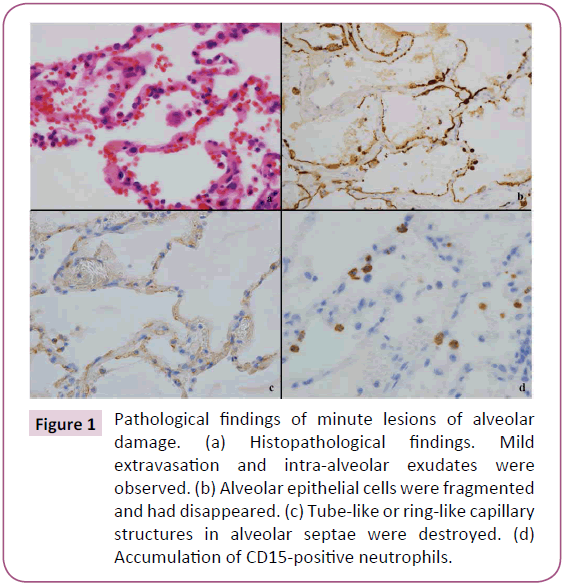
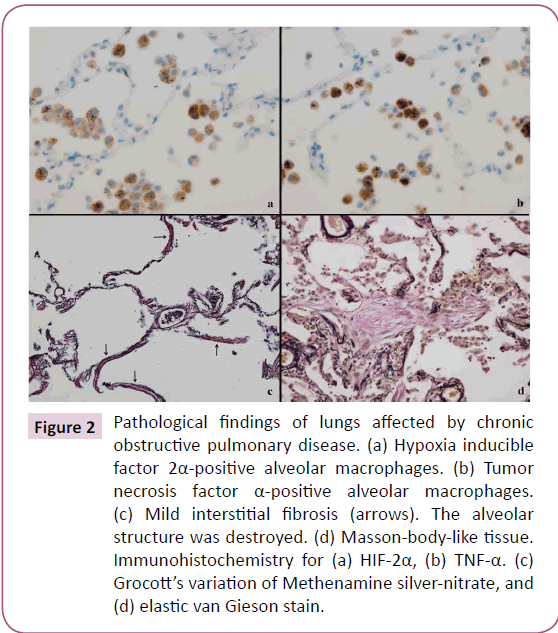
Mild extravasation of blood cells and intra-alveolar exudates were observed in architecturally normal lung tissue (Figure 1a). MLADs were detected in these areas. MLADs were microscopic foci of inflammatory lung injury where alveolar epithelial cells had fragmented and disappeared from the surface of the alveoli, leaving cytokeratin-positive cell debris (Figure 1b) and ring-like or tube-like capillary structures had disappeared from the alveolar septae (Figure 1c). HIF-2α+ macrophages (Figure 2a) and TNF-α+ macrophages (Figure 2b) were detected in and around the MLADs, and neutrophils had accumulated in the alveolar septa and spaces (Figure 1d). Up to seven alveoli were involved with each MLAD. Hyaline membranes were not detected in MLADs. Bacteria or inclusion bodies indicating viral infection were not detected in and around the MLADs.
Figure 1: Pathological findings of minute lesions of alveolar damage. (a) Histopathological findings. Mild extravasation and intra-alveolar exudates were observed. (b) Alveolar epithelial cells were fragmented and had disappeared. (c) Tube-like or ring-like capillary structures in alveolar septae were destroyed. (d) Accumulation of CD15-positive neutrophils.
Figure 2: Pathological findings of lungs affected by chronic obstructive pulmonary disease. (a) Hypoxia inducible factor 2α-positive alveolar macrophages. (b) Tumor necrosis factor α-positive alveolar macrophages. (c) Mild interstitial fibrosis (arrows). The alveolar structure was destroyed. (d) Masson-body-like tissue. Immunohistochemistry for (a) HIF-2α, (b) TNF-α. (c) Grocott’s variation of Methenamine silver-nitrate, and (d) elastic van Gieson stain.
Mild interstitial fibrosis (Figure 2c) and isolated Masson body-like tissues (Figure 2d) were observed around MLADs. The alveolar structure around areas of interstitial fibrosis was destroyed, and Masson body-like tissues were small in size and number.
Frequency of pathological findings
The results are shown in Table 1. TNF-α+ macrophages and HIF-2α+ macrophages were detected in 100% of patients in the smoking group with or without COPD, and in 2 of 10 patients in the non-smoking group with COPD. The number of patients with HIF-2α+ macrophages and TNF-α+ macrophages were significantly larger in the COPD smoking group and non-COPD smoking group than in the non-COPD non-smoking group (all P<0.001). These cells persisted after smoking cessation in 21 of 35 (60%) patients with COPD and 5 of 25 (20%) patients without COPD. The numbers of neutrophils in alveolar septae and alveolar spaces were significantly larger in the COPD smoking group and non-COPD smoking group than in the non-COPD nonsmoking group (COPD smoking group vs. Non-COPD non-smoking group, P=0.001, others, P<0.001). The numbers of neutrophils in alveolar septae and alveolar spaces were tend to be larger in and around MLADs [median (quartiles), alveolar septae; 254(167, 272), alveolar space; 128(86, 151)] than those in lung tissues located away from MLADs. MLADs were detected in 11 patients with COPD (smoking-6; smoking cessation-3; no smoking-2), and TNF-α+ macrophages and HIF-2α+ macrophages were observed in all of these patients. A long-acting beta2-agonist, a long-acting muscarinic antagonist or both were administered to 5 of the 11 patients. Masson body-like tissues were observed in the COPD groups and in patients without COPD in the smoking group. Mild interstitial fibrosis was detected in all groups. Nodular granulation tissue was not detected in patients with COPD.
Discussion
The data obtained by the present investigation suggest the following:
1). HIF-2α+macrophages and TNF-α+macrophages induced by hypoxia caused by smoking play an important role in the recruitment of neutrophils,
2). MLADs play an important role in the development of subsequent full-scale emphysema.
In the present study, HIF-2α+macrophages and TNF- α+macrophages were detected in 100% of smoking patients with or without COPD. The numbers of neutrophils in alveolar septae and alveolar spaces were significantly larger in the COPD smoking group and non-COPD smoking group than in the non-COPD nonsmoking group. Macrophages in ischemic disease sites accumulate both HIF-1α+ and HIF-2α+ [12,13]. HIF activity stimulates the production and release of pro-inflammatory cytokines such as TNF-α and interleukin-1 [14]. It is widely believed that proinflammatory cytokines released by macrophages in the alveolar lumen cause neutrophils to adhere to capillaries and extravasate into the alveolar space [15]. These data and findings suggest that hypoxia induced by smoking triggers an innate immune response and that HIF-2α+macrophages and TNF-α+macrophages induced by hypoxia play an important role in the recruitment of neutrophils in smoking patients.
In this study, we found MLADs in lungs with stable COPD. MLADs were restrictedly detected in lungs where HIF-2α+ alveolar macrophages and TNF-α+ alveolar macrophages were present. The numbers of neutrophils in alveolar septae and alveolar spaces were tending to be larger in and around MLADs than in lung tissues located away from MLADs. Alveolar epithelial cells and capillary endothelial cells in MLADs were injured. The alveolar structure was destroyed and mild interstitial fibrosis was observed in alveolar septae. As mentioned above, neutrophils seemed to be recruited into air spaces by proinflammatory cytokines released by HIF-2α+ alveolar macrophages and TNF-α+ alveolar macrophages. It is widely believed that neutrophils recruited into the alveolar space undergo activation by proinflammatory cytokines. Activated neutrophils release a variety of products (such as oxidants and proteases) that contribute to tissue damage [15]. These findings and data seem to indicate that MLADs develop in lungs in which large numbers of neutrophils have been recruited, probably by deterioration of hypoxia. Our present data seem to support the previous hypothesis that unopposed and increased elastolytic activity leads to destruction of elastic tissue in the walls of distal airspaces, eventually terminating in full-scale emphysema [9].
Masson body-like tissues estimated to be organizations of exudates as well as mild interstitial fibrosis estimated to represent the fibroproliferative phase of MLADs were observed in patients with COPD and smoking patients without COPD. A long-acting beta2-agonist, a long-acting muscarinic antagonist or both were administered to 5 of the 11 patients with MLADs. Although, these findings were not hallmarks of COPD, they seem to indicate that during the clinical course, the inflammatory lung injury continued to progress in spite of treatment, eventually leading to emphysema.
MLADs were detected in both patients with idiopathic pulmonary fibrosis [11] and patients with COPD. Nodular granulation tissue (the fibroproliferative phase of MLADs) was observed in all patients with idiopathic pulmonary fibrosis and is considered an important mechanism of lung remodeling [11]. In contrast, nodular granulation tissues were not observed in patients with COPD. We speculate that these findings might reflect the results of pulmonary function testing in patients with COPD and idiopathic pulmonary fibrosis.
We acknowledge certain limitations of this study. First, we could not find any papers discussing the presence of HIF- 2α+macrophages, TNF-α+macrophages and MLADs in lungs of stable COPD patients. We could not compare our results with those of other reports. Second, the number of examining patients was small. Thus, the reported results may not be broadly representative. However, HIF-2α+macrophages and TNF-α+macrophages were detected in all smoking patients with or without COPD. Accordingly, our present pathological findings were at least a part of pathological findings of inflammatory lung injury in patients with COPD. Therefore, we believe that future studies are important for understanding the pathobiological mechanisms of inflammatory lung injury in COPD patients.
Conclusion
COPD is usually a progressive neutrophilic inflammatory airway disorder that develops after long-term exposure to external stresses. Recruitment of neutrophils is reportedly mediated by a variety of molecular signals (such as microbial breakdown products and the chemokines). However, we found no reports describing the histopathologic features underlying the neutrophil-associated lung injury in lungs of patients with COPD. The results of the present investigation suggest the followings: (1). HIF-2α+macrophages and TNF-α+macrophages induced by hypoxia caused by smoking play an important role in the recruitment of neutrophils, (2). MLADs play an important role in the development of subsequent full-scale emphysema.
Acknowledgment
The authors sincerely express their appreciation to Dr. Shinichi Toyabe (Crisis Management Office, Niigata University) for his statistical analysis.
We thank Angela Morben, DVM, ELS, from Edanz Group (www. Edanzediting.com/ac) for editing a draft of this manuscript.
References
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, et al. (2007) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176: 532-55.
- Rennard SI, Vestbo J (2006) COPD: The dangerous underestimate of 15%. Lancet 367: 1216-19.
- Doll R, Peto R, Boreham J, Sutherland I (2004) Mortality in relation to Smoking: 50 years’ observation on male British doctors. J Epidemiol Community Health. 328: 159.
- Mannino DM (2002) COPD: Epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest 121: 121s-126s.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, et al. (2013) Global strategy for the diagnosis, management, and prevention of Chronic Obstructive Pulmonary Disease. Gold executive summary. Am J Respir Crit Care Med. 187: 347-65.
- Stanescu D, Sanna A, Veriter C, Kostianev S, Calcagni PG, et al.( 1996) Airways obstruction, Chronic expectoration, and rapid decline of FEV1 in smoker are associated with increased levels of sputum neutrophils. Thorax 51: 267-71.
- O’Donnell RA, Peebles C, Ward JA, Daraker A, Angco G, et al. (2004) Relationship between peripheral airway dysfunction, airway obstruction, and neutrophilic inflammation in COPD. Thorax. 59: 837-42.
- Grabcanovic-Musija F, Obermayer A, Stoiber W, et al. (2015) Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with air flow limitation. Respiratory Research 16: 59-70.
- Cosio MG, Saetta M, Agusti A (2009) Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med 360: 2445-54.
- Hunninghake GW, Crystal RG (1983) Cigarette smoking and lung destruction. Accumulation of neutrophils in the lungs of cigarette smokers. Am Rev Respir Dis 128: 833-38.
- Emura I, Usuda H, Togashi K, Satou K (2015) Minute lesions of alveolar damage in lungs of patients with stable idiopathic pulmonary fibrosis. Histopathology 67: 90-95.
- Burke B, Tang N, Corke KP (2002) Expression of HIF-1 alpha by human macrophages: Implications for the use of macrophages in hypoxia-regulated cancer gene therapy. J Pathol 196: 204-12.
- Elzschig HK, Carmeliet P (2011) Hypoxia and inflammation. N Engl J Med 364: 656-65.
- Nizet V, Johnson RS (2009) Interdependence of hypoxic and innate immune responses. Nat Rev Immunol 9: 609-17.
- Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. New Engl J Med 342: 1334-49.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences