Mature Cystic Teratoma of the Lung: A Case Report
Gómez Sáenz JT, Gérez Callejas MJ, Zangróniz Uruñuela MR, Díaz Ramírez M, Ibáñez Leza A, ldaz Vaca S
DOI10.21767/2572-5548.100007
Gómez Sáenz JT1*, Gérez Callejas MJ2, Zangróniz Uruñuela MR1, Díaz Ramírez M1, Ibáñez Leza A3 and Aldaz Vaca S4
1Family Phisician, Nájera’s Health Center, Nájera, La Rioja
2Family Phisician, Emergency Service 061, Hospital San Pedro. Logroño, La Rioja
3Resident of Family Phisician, 4th Year, Joaquin Elizalde Health Center, Logroño, La Rioja
4Resident of Family Phisician, 1st Year, Joaquin Elizalde Health Center, Logroño, La Rioja
- *Corresponding Author:
- Gómez Sáenz JT
Family Phisician, Nájera’s Health Center, Nájera (La Rioja), Spain
Tel: (254) 756-7091
E-mail: jtgomez@riojasalud.es
Received Date: January 24, 2016 Accepted Date: February 15, 2016 Published Date: February 22, 2016
Citation: Gómez Sáenz JT, Gérez Callejas MJ, Zangróniz Uruñuela MR, et al. (2016) Mature Cystic Teratoma of the Lung: A Case Report. Chron Obstruct Pulmon Dis 1:7. doi: 10.21767/2572-5548.100007
Copyright: © 2016 Gómez Sáenz JT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Teratomas are tumours composed of tissues derived from more than one germ cell line. Intrathoracic teratomas are usually seen in the mediastinum and very rarely in the lung. We report a case of a giant pulmonary teratoma occupying most of the right hemithorax in a 5-year-old female child with complaints of chest pain and low grade fever for the last 5 days. Patient was successfully operated with no recurrence in a 9 months follow up.
Introduction
Teratoma is a common tumour of the mediastinum but is rarely found in the lung, with less than a hundred cases published up to date [1,2].
Intrapulmonary teratoma originates from the totipotential cells or one, or more, of the three germinative layers, which can differentiate into any tipe of tissue [1,3].
In this article we describe a giant lung teratoma that, occupying most of the right lung, was solved by complete resection.
Case Report
A 5-year-old female complained of right-sided chest pain that increased with movements and low grade fever for the last 5 days.
Physical examination showed a haggard girl with good general health, axillary temperature of 37.6 degrees, heart rate of 120/min and respiratory rate of 20/min; oxygen saturation on room air was 98%.
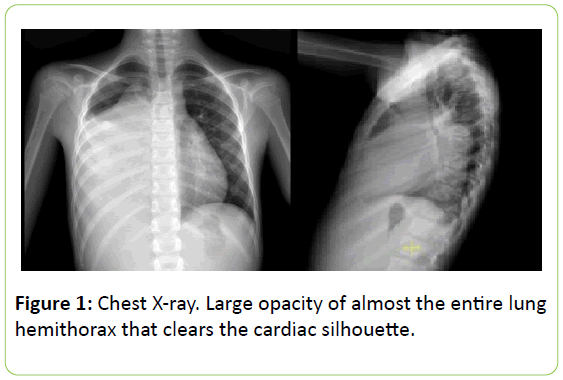
Auscultation of the chest revealed decreased air entry on the lower two thirds of right lung with tubal murmur and dullness. A chest X-ray showed a large opacity of almost the entire lung hemithorax that cleared the cardiac silhouette (Figure 1). With suspected diagnosis of pneumonia the patient was derived to the hospital. The girl was not vaccinated against pneumococcus, rotavirus or chicken pox.
At the emergency department blood tests showed 14.600/ ml3 white blood cells with 77.2% of neutrophils and C-reactive protein of 92 mg/dl.
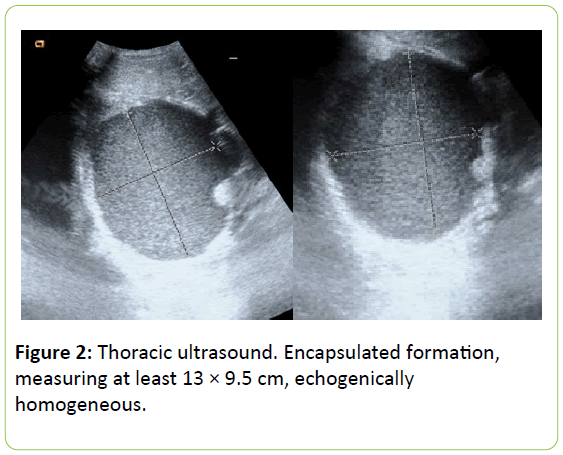
A thoracic ultrasound was performed showing a small sheet of pleural effusion and an encapsulated formation, measuring at least 13 × 9.5 cm, echogenically homogeneous with thick material without internal frame except for the existence of an echogenic pole 21 mm in the medial side that seems to point to hilar and contacting on with the anterior thoracic that impressed of cystic formation (Figure 2).
Serological test for pneumococcal, atypical pneumonia and hydatidosis were negative. At paediatric hospitalization treatment with cefotaxime and azythromycin was established, with no fever after 24 hours. Cefotaxime was discontinued because of a skin rash.
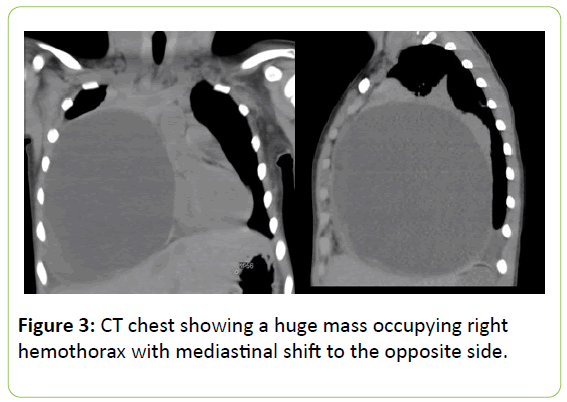
A Computed Tomography Chest (CT) was advised which revealed a huge mass occupying the lower two thirds of right lung with mediastinal shift to the opposite side (Figures 3 and 4).
A large well-defined cystic formation of 9 × 10 × 13 cm with a thin and ovoid wall is seen. It exerts mass effect, moving the right hemidiafragm caudally, shifting the mediastinum to the left and causing total atelectasis of the right middle lobe and partial atelectasis of right upper and lower lobes.
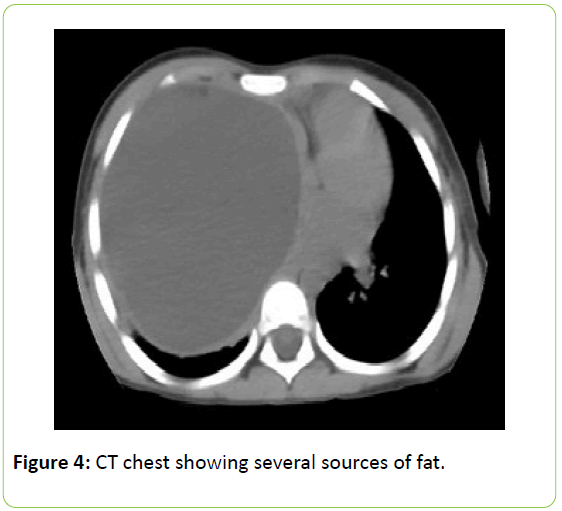
It has a predominantly hypodense (18 UH medium) and homogeneous content with two small pockets of fat density adjacent to and anterior mediastinum and flow of higher density (30 UH medium).
The presence of two sources of fat in the cyst point to a teratoma. Due to the large size of the lesion it is difficult to know where it originates but the upper contacts the thymus in the anterior superior mediastinum.
Tumor markers (α-fetoprotein and β-human chorionic gonadotropin) were both normal. Mantoux test was negative. With a suspicion diagnosis of mature cystic teratoma of the lung the patient was sent to the Surgery reference hospital where a thoracotomy was performed, removing the entire mass.
The histopatological examination reports the presence of pancreatic parenchyma, gastrointestinal stromal-like structures and Paneth’s cells.
There is a cystic respiratory squamous epithelium lining with skin and thymic fragments without immature tissues. It all supports a cystic mature teratoma with extensive areas of ischemic necrosis.
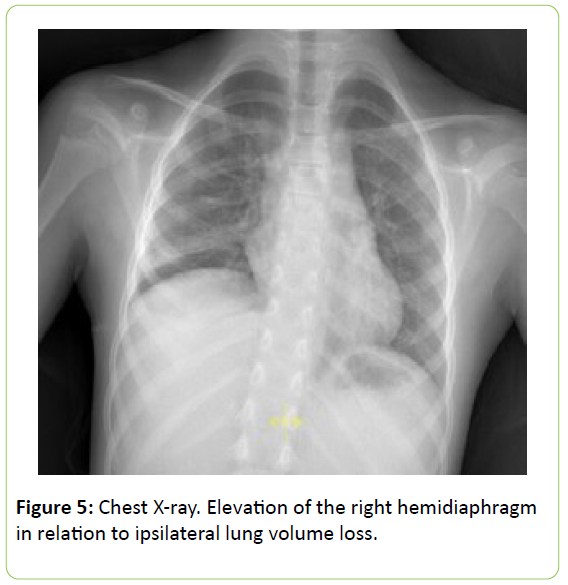
The post-operative course was unevenful and the patient was discharged in stable condition on 10th post-operative day. X-ray shows a faint elevation of the right hemidiaphragm in relation to ipsilateral lung volume loss (Figure 5).
Up to date the patient is asymptomatic with no relapse after 9 months from surgery.
Discussion
Teratomas or germ cell tumours have a worldwide incidence of about one in 4000 born living [4]. They are predomintantly found in gonads, while the anterior mediastinum is the most common extragonadal site [5,6]. In decreasing order, the sacrococcygeal regium (40%) ovaries (25%), testicles (12%), brain (5%), neck and mediastinum (18%) may be involved [4,7]. The mediastinum is the second most common site for teratomas in the paediatric population, after thymomas, accounting for 8% of all mediastinal tumours [6-8]. More than 80% of them are located in the anterior mediastinum [4,8,9], space located between retrosternum and the anterior face of heart and great vessels that includes thymus, lymph nodes as well as fat and connective tissue [9]. Teratomas are equally present in boys and girls and seems to be more prevalent in adult males [8,10].
It is presumed that intrathoracic teratomas develop, in relation to the thymus, as derivatives of the third pharingeal pouch with a predilection for the upper lobes [7,10,11]. They come from pluripotent cells and make up from one or more of the three germ cell layers [1,4,5]. From ectoderm they can have skin, hair and sebaceous glands; from mesoderm, connective tissue, cartilage, muscle, bone, nervous o lymphatic tissues and from endoderm, pancreatic tissue, respiratory and intestinal epithelium [3,12]. Most of mediastinum teratomas are cystic, benign and well defined. Solid forms are less common and usually malignant. The last are bigger than benign forms and invade adjacent structures [12].
Teratomas can be classified as mature (well differentiated and with at least two tissues derived from germ cell layers), inmature (same tissues than mature but with embryonic tissues) and teratomas with malignant transformation that may involve up to 30% of teratomas [3,7,13]. Malignant teratoma is defined by the presence of immature tissue within the teratoma rather than the presence of metastases or infiltration [7].
Although mediastinal teratomas are far from being a rarity, primary pulmonary teratomas are extremely uncommon [2]. Pulmonary teratomas are mostly composed of mature, cystic, somatic tissues and present as encapsulated masses with a thin wall containing liquid tissues, fat, calcifications or any combination of this elements [4].
Almost one half of patients have no sign or symptoms when the mass is discovered and the majority of them are diagnosed in the first two decades [7,11,14]. Younger patients are more prone to be symptomatic [6,14]. Chest pain is the most frequent symptom and can be found in 52% of the cases [7,15]. Other symptoms are hemoptysis, cough, back or shoulder pain, dysnea, fever, pleural effusion or bulging of the chest wall [1,11,14]. Symptons can also derive from the pressure exerted on the surrounding tissues, although superior vena cava syndrome is very uncommon [10,13,16]. The most specific sign is the expectoration of hair or trichoptysis due to bronchial communication that appears in one in ten patients [2,7,11]. Teratomas can also produce digestive or proteolytic enzymes that made them prone to rupture to skin or bronchus [11,13]. Some tumor markers can be helpful in differencial diagnosis. Inmature teratomas in more than 60% show high α- fetoprotein and β-human chorionic gonadotropin (βHCG) levels while in seminomas α-fetoprotein is normal and βHCG is elevated in 10% of the cases [6,8,9].
Radiologically, lesions are tipically cystic, well defined, often with focal calcifications [7]. Cystic are close to heart and great vessels and a peripheral calcification, bone or teeth tissues can be found [13]. The finding of a fat/liquid level is highly specific for a teratoma diagnosis [13,16]. CT is the diagnostic technique of election. The characteristic image is a heterogeneous mass with well-defined edges and lobed cyst. CT scans show discrete areas of soft tissue, high local fat content, punctate calcification, or a combination of them and is very helpful to distinguish between a ruptured and an unruptured teratoma [2,7,14]. It is described the presence of fat in varying proportions in 90% of mature teratomas [16]. Magnetic resonance is only useful if an affectation vascular or neural structures is suspected or in cases of patients with kidney failure or allergy to iodinated contrast [9,11,15].
Differential diagnosis must be done with endothoracic goiter, lymphoma, thymic lesions (thymoma, cancer, hyperplasia, cysts, thymolipoma) and other germ cell tumors [16,17].
Surgical ressection is the only effective way to treat teratoma [5]. The prognosis of a mature cystic teratoma is excellent if it can be removed [4,12]. The 6 year relapse-free survival for completed resected teratoma was 96% as compared to 55% for incompleted in a review of 153 children with no testicular mature teratomas [4]. Inmature teratomas without malignacy transformation have also a good prognosis. However the prognosis of malignat teratoma is ominous with more than 60% of patients dying within a 6-month follow-up [7,12]. The size of the tumour or a rapid growth is not a sign of malignancy [1].
Conclusion
Intrapulmonary teratomas are very unsual tumours. They derive from pluripotent cells and make up from one or more of the three germ cell layers. We can classify them as mature or immature in case of including embrionic tissues. A giant teratoma involving most of the right hemithorax is exceptional and noteworthy. Complete resection is the ideal treatment with a good long-term prognosis.
References
- Medeiros CWDL, Kondo W, Dyckyj MT, Suzuki N (2001)Teratomaintrapulmonar. J Pneumol 27:272-274.
- Turna A, Özgül A, Kahraman S, Gürses A, Fener N, (2009) Primary Pulmonary Teratoma: Report of a Caseand the Proposition of «Bronchotrichosis»as a New Term. Ann Thorac Cardiovasc Surg 15:247-249.
- BernotRamírez D, MederosCurbelo ON, Coto Borges R (1997) Teratomaquístico del pulmón. Rev Biomed 8:209-210.
- Anand S, Longia S, Agarwal N, Maheshawari M, Apte A (2010) Mature mediastinalteratoma. A rare cause of recurrent respiratory distress. Peoples J Sci Res 3:33-35.
- Zhao H, Zhu D, Zhou Q (2014) Complete resection of a giant mediastinalteratoma occupying the entire right hemithorax in a 14-year-old boy. BMC Surg 14:56.
- Farías SB, Molise MC, Fernández Bedoya I, Frontera DA, Litvak E (2006) Teratomamediastínico: causapocofrecuente de dificultadrespiratoriaaguda en lactantes. Arch Argent Pediatría104:261-264.
- Mondal SK, Das Gupta S (2012) Mature cystic teratoma of the lung. Singapore Med J 53:e237.
- Cadavid AL, Berrios GC, García BC (2009) Caso Clínico Radiológico Pediátrico. Rev Chil Enfermedades Respir 200925.
- Monteiro R, Alfaro TM, Correia L, Simao A, RobaloCordeiro C, et al. (2011) Massasmediastínicas. Análise de umacasuística. Acta Med Port 24:899-904.
- Yalagachin GH (2013) Anterior MediastinalTeratoma- A Case Report with Review of Literature. Indian J Surg. junio de 75:182-184.
- Dar RA,Mushtaque M, Wani SH, Malik RA (2011) Giant Intrapulmonary Teratoma: A Rare Case. Case Rep Pulmonol1-3.
- Valdivia Salas MM, Ruiz López FJ (2007) Mujerjoven con toscrónica. Rev PatolRespir10:150-152.
- Gurdián GM. Casoclínico-radiológico no 12.
- Serraj M, Lakranbi M, Ghalimi J, Ouadnouni Y, Smahi M (2013) Mediastinal mature teratoma with complex rupture into the lung, bronchus and skin: a case report. World J Surg Oncol 11:125.
- Call Caja S, Rami Porta R, Serra Mitjans M, BeldaSanchís J, Barreiro López B (2007)Lesiónquísticamediastinica de histologíaindeterminada. Rev PatolRespir10: 156-158.
- BandrésCarballo B, Parra Gordo ML, González Sendra MJ, Velasco Ruiz M, Olivera Serrano MJ (2010) Teratomamaduromediastínico. Rev Med Gen Fam127:198-200.
- Godoy R, López P, León P, Callejas J,Ceballos JC (2009) Linfangiomamediastínico. Rev PatolRespir12:181-182.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences