Individualized Care in COPD: Updated Guidelines from Revised GOLD 2017 Report
Kavita Ratarasarn, Jatan Shah and Nevin Uysal-Biggs
DOI10.21767/2572-5548.100027
Kavita Ratarasarn1,2*, Jatan Shah1,2 and Nevin Uysal-Biggs1,2
1Medical College of Wisconsin, W Wisconsin Av, Milwaukee, WI, USA
2CJ Zablocki Veterans Affairs Medical Center, W National Ave, Milwaukee, WI, USA
- *Corresponding Author:
- Ratarasarn K
Medical College of Wisconsin
CJ Zablocki Veterans Affairs Medical Center
Milwaukee, WI, USA
Tel: 4149557043
Fax: 4149556211
E-mail: kratarasarn@mcw.edu
Received date: October 04, 2017; Accepted date: November 21, 2017; Published date: November 28, 2017
Citation: Ratarasarn K, Shah J, Uysal-Biggs N (2017) Individualized care in COPD: Updated guidelines from revised GOLD 2017 Report. Chron Obstruct Pulmon Dis 2: 27. doi:10.21767/2572-5548.100027
Copyright: © 2017 Ratarasarn K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Chronic obstructive lung disease (COPD) is a major cause of morbidity and mortality around the world. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) program provides evidence based guidelines to optimize management of patients with COPD. The Global Strategy for Diagnosis, Management and Prevention of COPD 2017 report incorporates many changes based on updated research published through 2016. It includes new strategies to promote individualized care with the goal of improving patient-centered outcomes. The new definition, revised combined assessment tool and the new approaches to management of COPD are highlighted in our review.
Keywords
COPD assessment; Gold 2017 guidelines; Gold 2017 update; COPD treatment; COPD outpatient management; Global initiative for chronic obstructive lung disease; Chronic obstructive pulmonary disease
Introduction
Chronic obstructive lung disease (COPD) is a major cause of chronic morbidity and is the fourth leading cause of mortality worldwide [1]. In the USA, it is the third leading cause of death as well as the second leading cause of reduced disabilityadjusted life years (DALYs) [2,3]. The disease burden is projected to continue to rise worldwide because of ongoing exposure to COPD risk factors and aging of the population [4]. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) program was initiated in 1988 to optimize management of COPD and promote prevention worldwide. In 2001, it published its first report Global Strategy for Diagnosis, Management and Prevention of COPD to provide clinicians with evidence-based management guidelines. The GOLD Science Committee periodically revises the report based on new research findings. The 2017 report represents the fourth major revision based on new information in publications through 2016 related to pathophysiology, diagnosis, assessment and approaches to management of COPD [5]. Our review highlights the GOLD updates on outpatient management of stable COPD.. These updates are of relevance to primary care providers as well as pulmonary specialists.
Diagnosis of COPD
GOLD’s definition of COPD was changed in its 2017 report to “A common, preventable and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases” [5]. The prior definition referenced inflammation, comorbidities and exacerbations but this new simplified definition brings focus to the typical clinical picture at time of presentation. COPD should be considered in any individual who has dyspnea, chronic cough, chronic sputum production or recurrent lower respiratory tract infections and a history of exposure to risk factors. The updated list of risk factors in GOLD 2017 report adds genetic and individual factors (Table 1).
|
Host factors |
|
Adapted from “From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease [GOLD] 2017”. Copyright 2017 by Global Initiative for Chronic Obstructive Lung Disease. |
Table 1: Risk factors for COPD.
Since symptoms associated with COPD are nonspecific and overlap with cardiac, systemic and other pulmonary disorders, spirometry is required to confirm COPD.
Spirometry reliably measures forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC). The ratio of these two measurements (FEV1/FVC) is used to determine presence or absence of airflow limitation. As per GOLD guidelines, a postbronchodilator FEV1/FVC ratio of <0.70 identifies persistent airflow limitation. This differs from ATS guidelines that recommend using ratio less than lower limits of normal (FEV1/FVC <LLN) to identify airflow limitation [6]. While more data are needed to determine ideal diagnostic spirometry criterion, GOLD favors use of fixed ratio for its simplicity and consistency. However, clinicians should be mindful that using FEV1/FVC ratio <0.70 to define airflow limitation may result in over-diagnosis in the older individuals, and under-diagnosis in adults <45 years, especially in the presence of mild disease [7,8].
Initial COPD Assessment
The goals of initial COPD assessment are to determine the severity of airflow limitation, the impact of disease on the patient’s health status, and the risk of future events (such as exacerbations, hospital admissions, or death). Comorbidities contribute significantly to morbidity and mortality associated with COPD. Thus, assessment of all these factors is required to provide individualized treatment for each patient.
Assessment of air flow limitation: Classification of severity of airflow limitation is based on post-bronchodilator FEV1 (Table 2). The degree of reversibility on post-bronchodilator spirometry does not add to differential diagnosis or predict response to therapy [9].
| GOLD 1 | Mild | FEV1 ≥ 80% predicted |
| GOLD 2 | Moderate | FEV1 50% to < 80% predicted |
| GOLD 3 | Severe | FEV1 30% to <50% predicted |
| GOLD 4 | Very severe | FEV1<30% predicted |
| Adapted from “From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017”. Copyright 2017 by Global Initiative for Chronic Obstructive Lung Disease. FEV1: Forced expiratory volume in 1 second. |
||
Table 2: GOLD Classification of airflow limitation based on post-bronchodilator FEV1.
Assessment of symptoms: Severity of airflow limitation has a weak correlation with symptoms and impairment of a patient’s health-related quality of life. Several tools are available to specifically measure burden of disease. GOLD favors the use of the Modified British Medical Research Council (mMRC) dyspnea scale (Table 3) and COPD assessment test (CAT) (Table 4), primarily for their ease of use in clinical practice [10,11]. CAT is favored over mMRC dyspnea scale as it allows a more comprehensive assessment of symptoms beyond just breathlessness. mMRC grade 0-1 and CAT score <10 indicate a low-symptom profile.
| mMRC grade 0 | I only get breathless with strenuous exercise | ÃÆâÃâââ¬âÃâá |
| mMRC grade 1 | I get short of breath when hurrying on the level or walking up a light hill | ÃÆâÃâââ¬âÃâá |
| mMRC grade 2 | I walk slower than other people of the same age on the level because of breathlessness, or I have to stop for breath when walking on my own pace on the level | ÃÆâÃâââ¬âÃâá |
| mMRC grade 3 | I stop for breath after walking about 100 meters or after a few minutes on the level | ÃÆâÃâââ¬âÃâá |
| mMRC grade 4 | I am too breathless to leave the house or I am breathless when dressing or undressing | ÃÆâÃâââ¬âÃâá |
Table 3: Modified Medical Research Council (mMRC) Dyspnea Scale.
| For each item below, place a mark (X) in the box that best describes you currently. Be sure to only select one response for each questions | ||||
| Example | I am very happy | ÃÆâÃâââ¬ÅÃâêÃÆâÃâââ¬ËÃâàÃÆâÃâââ¬ËÃâáÃÆâÃâââ¬ËÃââÃÆâÃâââ¬ËÃâãÃÆâÃâââ¬ËÃâä | I am very sad | Score |
| I never cough | ÃÆâÃâââ¬ÅÃâêÃÆâÃâââ¬ËÃâàÃÆâÃâââ¬ËÃâáÃÆâÃâââ¬ËÃââÃÆâÃâââ¬ËÃâãÃÆâÃâââ¬ËÃâä | I cough all the time | ÃÆâÃâââ¬âÃâá | |
| I have no phlegm (mucus) in my chest at all | ÃÆâÃâââ¬ÅÃâêÃÆâÃâââ¬ËÃâàÃÆâÃâââ¬ËÃâáÃÆâÃâââ¬ËÃââÃÆâÃâââ¬ËÃâãÃÆâÃâââ¬ËÃâä | My chest is completely full of phlegm | ÃÆâÃâââ¬âÃâá | |
| My chest does not feel tight at all | ÃÆâÃâââ¬ÅÃâêÃÆâÃâââ¬ËÃâàÃÆâÃâââ¬ËÃâáÃÆâÃâââ¬ËÃââÃÆâÃâââ¬ËÃâãÃÆâÃâââ¬ËÃâä | My chest feels very tight | ÃÆâÃâââ¬âÃâá | |
| When I walk up a hill or one flight of stairs I am not breathless | ÃÆâÃâââ¬ÅÃâêÃÆâÃâââ¬ËÃâàÃÆâÃâââ¬ËÃâáÃÆâÃâââ¬ËÃââÃÆâÃâââ¬ËÃâãÃÆâÃâââ¬ËÃâä | When I walk up a hill or one flight of stairs I am very breathless | ÃÆâÃâââ¬âÃâá | |
| I am not limited doing any activities at home | ÃÆâÃâââ¬ÅÃâêÃÆâÃâââ¬ËÃâàÃÆâÃâââ¬ËÃâáÃÆâÃâââ¬ËÃââÃÆâÃâââ¬ËÃâãÃÆâÃâââ¬ËÃâä | I am very limited doing activities at home | ÃÆâÃâââ¬âÃâá | |
| I am confident leaving my home despite my lung condition | ÃÆâÃâââ¬ÅÃâêÃÆâÃâââ¬ËÃâàÃÆâÃâââ¬ËÃâáÃÆâÃâââ¬ËÃââÃÆâÃâââ¬ËÃâãÃÆâÃâââ¬ËÃâä | I am not at all confident leaving my home because of my lung condition | ÃÆâÃâââ¬âÃâá | |
| I sleep soundly | ÃÆâÃâââ¬ÅÃâêÃÆâÃâââ¬ËÃâàÃÆâÃâââ¬ËÃâáÃÆâÃâââ¬ËÃââÃÆâÃâââ¬ËÃâãÃÆâÃâââ¬ËÃâä | I don’t sleep soundly because of my lung condition | ÃÆâÃâââ¬âÃâá | |
| I have lots of energy | ÃÆâÃâââ¬ÅÃâêÃÆâÃâââ¬ËÃâàÃÆâÃâââ¬ËÃâáÃÆâÃâââ¬ËÃââÃÆâÃâââ¬ËÃâãÃÆâÃâââ¬ËÃâä | I have no energy at all | ÃÆâÃâââ¬âÃâá | |
| Total Score | ||||
| Adapted from: “Development and first validation of the COPD Assessment Test. Eur Respir J (2009) 34: 648-654”. | ||||
Table 4: COPD assessment test.
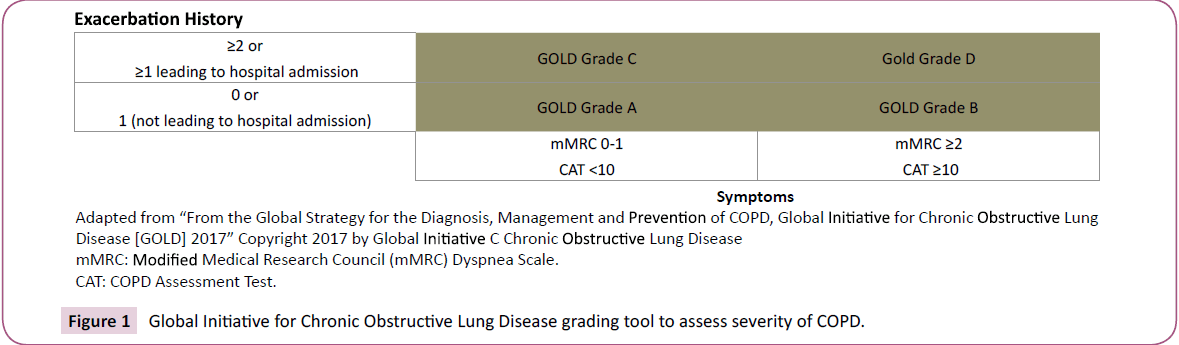
Assessment of risk for exacerbations: An exacerbation of COPD is defined as “Acute worsening of respiratory symptoms that result in additional therapy”. COPD exacerbations are associated with accelerated rate of decline in FEV1, deterioration in health status and risk of death [12,13]. The most reliable way of assessment future risk of exacerbations is to look at treated exacerbations in the past. Increased risk of exacerbations is defined as 2 or more exacerbations per year or 1 exacerbation leading to hospital admission. In patients not on inhaled steroid treatment, GOLD 2017 updated guidelines incorporate increased blood eosinophil count as a predictor of exacerbations [14,15]. However, prospective clinical trials are needed to establish blood eosinophil cut-off values that can be used in clinical decisions.
GOLD 2017 report recommends that most therapeutic decisions be based on ABCD assessment tool that grades severity of disease based on symptoms and risk for exacerbations. This tool is presented in (Figure 1).
Assessment of comorbidities
Common comorbidities associated with COPD include cardiovascular disease, skeletal muscle dysfunction, metabolic syndrome, osteoporosis, depression, anxiety and lung cancer [16-18]. Comorbidities can occur with any degree of airflow limitation. They diminish the quality of life, and increase hospitalization mortality rates; only 40% of patients with COPD die from it [19-21]. Therefore, clinicians should look for these conditions in all patients diagnosed with COPD, and treat them appropriately.
Comprehensive COPD evaluation protocol includes diagnosis, assessment of severity of airflow limitation, assessment of severity of symptoms, assessment of risk of exacerbations and identification of comorbidities that add to disease burden for each individual patient. (Figure 2).
Additional Testing
Imaging: Chest X-ray is used primarily to exclude alternative diagnoses. CT chest is not routinely recommended but is needed if considering invasive treatment for COPD.
Lung volumes and diffusion capacity: These tests may be considered to look for other conditions when the degree of air flow limitation does not explain dyspnea.
Oximetry and arterial blood gases (ABGs) measurement: Pulse oximetry should be performed for all stable patients with FEV1 <35% predicted or with clinical signs of respiratory failure and/or right heart failure. ABGs measurement is indicated for all patients with baseline pulse oximetry saturation level <92%.
Screening for alpha-1 antitrypsin (AAT) deficiency: Patients from areas with high prevalence of alpha-1 antitrypsin deficiency and with earlier presentation [<45 years with pan lobular emphysema] should be screened for AAT deficiency.
Exercise testing: Walking distance measured by the six-minute walk test or the shuttle walk test is used to assess degree of disability and mortality risk, and to monitor response to pulmonary rehabilitation [22-24].
Composite scores: The BODE (Body mass index, Obstruction, Dyspnea, and Exercise) index are easy to calculate and is a better predictor of survival than any of its single components [25].
Outpatient Management of COPD
Treatment of COPD is targeted at reducing symptoms, exacerbations, hospitalizations and mortality as well as improving overall health status. To achieve these goals, a multipronged management strategy that includes pharmacological as well as non-pharmacological interventions is required. Interventions should be tailored to the individual patient’s needs based on COPD assessment protocol outlined in Figure 2. Some key changes in management included in GOLD 2017 report are:
• The pharmacologic regimen is based primarily on GOLD ABCD grade of disease. The primary role for spirometry is confirmation of diagnosis, prognostication and follow-up assessment. Spirometry does have a more prominent role in therapeutic decisions if there is significant discrepancy between spirometry and symptoms or when invasive therapies for COPD are being considered.
• Treatment escalation and de-escalation strategies are outlined to accommodate shifts in degree of baseline symptoms and pattern of exacerbations.
• Invasive interventional therapy options are listed for selected patients with emphysema and hyperinflation.
Pharmacologic Therapy
Pharmacologic therapy is effective at reducing symptoms and exacerbations but has not been shown to modify the decline in lung function [12,26–29]. Many classes of medications are available to treat COPD as highlighted below. The mainstay of pharmacologic treatment in patients with COPD, however, continues to be inhaled bronchodilators (beta2-agonists and anti-muscarinic drugs) and inhaled corticosteroids (Table 5).
Beta2 agonists: Short acting beta2 agonists (SABA) as well as long acting beta2 agonists (LABA) have a defined role in management. Unlike asthma, there is no association between use of this class and increased mortality in COPD [30-37].
Antimuscarinic drugs: Short acting muscarinic antagonists (SAMAs) and long acting muscarinic antagonists (LAMAs) have an important role in management of COPD. LAMA (Tiotropium) has been shown to have greater effect on reducing exacerbations compared to LABA treatment [38,39]. Because of concerns regarding adverse cardiovascular effects associated with this class, FDA conducted a review in 2009 and concluded that LAMA (Tiotropium) added to other standard therapies had no effect on cardiovascular risk [40].
Steroids
Inhaled corticosteroids (ICS): In patients with moderate to very severe COPD and exacerbations, an ICS combined with a LABA is more effective than either component alone in improving lung function, health status and reducing exacerbations but it has no impact on survival [41-44]. Inhaled steroids may be more effective in reducing exacerbations in patients with eosinophilia [45,46].
Oral steroids: Oral glucocorticoids which play a significant role in the acute management of exacerbations have no role in the chronic daily treatment in COPD because of a lack of benefit and a high rate of systemic complications.
Phosphodiesterase-4 (PDE4) inhibitor: Roflumilast reduces moderate and severe exacerbations in patients with chronic bronchitis and severe COPD. However, it has more adverse effect than inhaled medications [47,48].
Antibiotics: Macrolides (azithromycin, erythromycin) prescribed for anti-inflammatory effects may reduce exacerbation rates but are associated with increased bacterial resistance and ototoxicity [49,50]. Impact beyond one-year to prevent COPD exacerbations is unknown.
Mucolytics
In COPD patients not on inhaled corticosteroids, carbocysteine and/or N-acetylcysteine may reduce exacerbations and improve health status [51].
Methylxanthine: Theophylline has modest bronchodilator effect but toxicity limits its usage. Its side effect profile includes arrhythmias and convulsion which can prove to be fatal.
Alpha1 antitrypsin (AAT) augmentation: Never or ex-smokers with FEV1 of 35-60% and AAT deficiency are considered most suitable for AAT augmentation therapy. Patients with FEV>=65% may also be considered if there is evidence of progressive lung disease despite other optimal therapy. In view of the cost of therapy and lack of evidence for much benefit, discussion with individual patient is recommended before initiation of therapy [52].
Non-pharmacologic Therapies
Smoking cessation: Smoking cessation is the only evidencebased intervention that slows the accelerated decline in lung function in people with COPD [53]. Interventions include counseling and pharmacological therapy.
While a clear relation exists between the intensity of counseling and smoking cessation, as little as three minutes of counseling can lead to successful cessation [54,55].
The pharmacological therapy options include nicotine replacement (gum, inhaler, nasal spray, transdermal patch, sublingual tablet, or lozenge), and medication such as varenicline, bupropion or nortriptyline. Concomitant use of counseling and pharmacological therapy increases effectiveness of intervention [56]. Role of e-cigarettes as nicotine replacement therapy is unclear based on currently available data.
In addition, avoidance of other risk factors such as occupational dusts, air pollutants, fumes and gases that contribute to airway disease should be emphasized.
Vaccination
All COPD patients should receive influenza vaccination. Influenza vaccination in patients with COPD reduces severe lower respiratory tract infections and mortality, and over time reduces risk of ischemic heart disease [57,58]. Pneumococcal vaccination is recommended per CDC guidelines. Specific effects of Pneumococcal vaccination on infections and mortality in patients with COPD is unclear [59,60].
Pulmonary rehabilitation: Pulmonary rehabilitation is defined as “A comprehensive intervention based on thorough patient assessment followed by patient-tailored therapies that include, but are not limited to, exercise training, education, selfmanagement intervention aiming at behavior change, designed to improve the physical and psychological condition of people with chronic respiratory disease and to promote the long-term adherence to health-enhancing behaviors.” [61] It is recommended for all patients that continue to be symptomatic after optimization of pharmacologic therapy. It can be conducted in inpatient or outpatient settings including the patient’s home. It is one of the most cost effective strategies to improve shortness of breath, health status and exercise tolerance across all grades of COPD severity [62-64].
Self-management interventions: Self-management interventions include training in self-recognition of exacerbations, coping with breathlessness, increasing physical activity and improving nutrition in malnourished individuals. Self-management interventions that include action plans for worsening symptoms decrease respiratory-related as well as all cause hospitalizations [65].
Invasive therapy: Recommendations for invasive therapies are included for the first time in the GOLD 2017 report. Bullectomy, bronchoscopic lung volume reduction (BLVR) and lung volume reduction surgery (LVRS) improve health outcomes in carefully selected subset of patients [66-69]. Extent and pattern of emphysema on high-resolution computed tomography (HRCT), presence of inter-lobar collateral ventilation, patient and provider preferences and local expertise are important considerations when considering invasive treatment options for patients.
For selected patients with very severe COPD, lung transplantation should be considered. Patients should be informed that it improves QOL but not survival [70,71].
Palliative care: The goal of palliative care is to prevent and relieve suffering, and to optimize quality of life [72].
Opiates, neuromuscular electrical stimulation (NMES) chest wall vibration (CWV) and fans blowing air onto the face, oxygen, pulmonary rehabilitation, and non-invasive ventilation all reduce breathlessness [73-79].
Cognitive behavioral therapy and mind-body interventions [e.g., mindfulness-based therapy, yoga, and relaxation] can reduce anxiety and dyspnea and improve lung function, exercise capacity and fatigue [80].
Nutritional supplementation supports weight gain, improved respiratory muscle strength and overall health-related quality of life in malnourished patients. Fatigue may be reduced by selfmanagement education, pulmonary rehabilitation, nutritional support and mind-body interventions [81,82].
Prediction of 6-month survival in patients with end-stage COPD is unreliable and therefore early discussion of advance care planning is recommended [83]. Discussing issues surrounding death with compassion can reduce anxiety for patients and their families and avoid unnecessary, unwanted and costly invasive approaches [84]. For patients with terminal illness, hospice services may be beneficial.
Oxygen and non-invasive positive pressure ventilation: Oxygen administration (> 15 hours per day) improves survival in patients with severe resting hypoxemia [85].
In patients with overlap syndrome (both COPD and obstructive sleep apnea), continuous positive airway pressure (CPAP) improves survival and reduces hospitalizations [86]. There is conflicting data on the use of non-invasive positive pressure ventilation (NIPPV) on survival and re-hospitalization in chronic hypercapnic COPD. NIPPV should always be managed by providers familiar with strategies and devices associated with management of hypercapneic respiratory failure.
Monitoring and Follow-up
Follow-up visits should include symptom evaluation as well as discussion of treatment regimen. For patients on inhalers, inhaler technique should be checked during each visit. There is no evidence for superiority of any one type of hand held device over others. For patients that are able to use their prescribed devices properly, nebulized therapy offers no additional advantage [87-89]. Thus, it is important to assess patient’s physical and medical conditions to determine the most suitable inhaler device for them. Spirometry is recommended at least once a year in all patients. If there is a clear worsening of symptoms, imaging may be indicated. Step up in therapy to prevent future exacerbations and step down in therapy in stable patients should be implemented as needed during the follow up visits (Table 5).
| GOLD Grade A | Single bronchodilator → alternative class of bronchodilator if needed → discontinue if appropriate |
| GOLD Grade B | LABA or LAMA → LABA/LAMA if symptoms persist |
| GOLD Grade C | LAMA → LABA/LAMA (preferred)* or LABA/ICS if exacerbations |
| GOLD Grade D | LABA/LAMA → LABA/LAMA/ICS if symptoms/exacerbations (preferred)* or LABA/ICS → LABA/LAMA/ICS if symptoms/exacerbations or LAMA → LABA/LAMA → LABA/LAMA/ICS if symptoms/exacerbations |
| If further exacerbations, consider: Roflumilast (FEV1<50%, chronic bronchitis) or Macrolide (former smoker) If stable on LABA/LAMA/ICS, consider de-escalation to LABA/LAMA |
|
| * LABA/LAMA is more effective than LABA or LAMA or ICS/LABA for decreasing exacerbations *LABA: Long-Acting Beta-Agonists *LAMA: Long-Acting Muscarinic Antagonists *ICS: Inhaled Corticosteroid : Forced expiratory volume in 1 second. Adapted from “From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017”. Copyright 2017 by Global Initiative for Chronic Obstructive Lung Disease. |
|
Table 5: Pharmacological treatment strategies by GOLD Grade.
Conclusion
COPD is a major cause of morbidity and mortality in the USA and around the world. Based on review of literature published through 2016, the GOLD 2017 report includes major changes in guidelines for assessment as well as treatment. All providers that treat patients with COPD should be aware of these updated strategies that promote individualized COPD care delivery targeted at symptom control, improved quality of life as well as reduction of COPD associated mortality.
References
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, et al. (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095-128.
- Hoyert DL, Xu J (2011) Deaths: Preliminary data for 2011. Natl Vital Stat Rep 61: 1-51.
- Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, et al. (2013) The state of US health, 1990-2010: Burden of diseases, injuries, and risk factors. JAMA 310: 591-608.
- Mathers CD, Loncar D (2016) Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3: 442.
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, et al. (2017) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med 195: 557-582.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, et al. (2005) Interpretative strategies for lung function tests. Eur Respir J 26: 948–968.
- van Dijk W, Tan W, Li P, Guo B, Li S, et al. (2015) Clinical relevance of fixed ratio versus lower limit of normal of FEV1/FVC in COPD: patient-reported outcomes from the CanCOLD cohort. Ann Fam Med 13: 41-48.
- Guder G, Brenner S, Angermann CE, Ertl G, Held M, et al. (2012) GOLD or lower limit of normal definition? A comparison with expert-based diagnosis of chronic obstructive pulmonary disease in a prospective cohort study. Respir Res 13:13.
- Hansen JE, Porszasz J (2014) Counterpoint: Is an increase in FEV1 and/or FVC ≥ 12% of control and ≥ 200 ml the best way to assess positive bronchodilator response? No. Chest 146: 538-541.
- Fletcher CM (1960) Standardised questionnaire on respiratory symptoms: a statement prepared and approved by the MRC committee on the aetiology of chronic bronchitis (MRC breathlessness score). Br Med J 2: 241-243.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, et al. (2009) Development and first validation of the COPD assessment test. Eur Respir J 34: 648-654.
- Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, et al. (2009) Randomised double blind placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: The ISOLDE trial. BMJ 320: 1297-1303.
- Jones PW (2009) Health status and the spiral of decline. COPD. 6: 59-63.
- Pascoe S, Locantore N, Dransfield M, Barnes NC, Pavord ID (2015) Blood eosinophil counts exacerbations and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: A secondary analysis of data from two parallel randomized controlled trial. Lancet Respir Med 3: 435-442.
- Siddiqui SH, Guasconi A, Vestbo J, Jones P, Agusti A, et al. (2015) Blood eosinophils: A biomarker of response to extrafine beclamethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 192: 523-525.
- Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL (2005) Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest 128: 2099-2107.
- Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, van Empel VP, et al. (2013) Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 187: 728-735.
- Chen W, Thomas J, Sadatsafavi M, FitzGerald JM (2015) Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Lancet Respir Med 3: 631-639.
- Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, et al. (2010) Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 11: 122.
- Mannino DM, Thorn D, Swensen A, Holguin F (2008) Prevalence and outcomes of diabetes hypertension and cardiovascular disease in COPD. Eur Respir J 31: 962-969.
- Smith MC, Wrobel JP (2014) Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis 9: 871–888.
- Celli B, Tetzlaff K, Criner G, Polkey M, Sciurba F, et al. (2016) The 6-minute Walk Test as a COPD Stratification Tool: Insights from the COPD Biomarker Qualification Consortium. Am J Respir Crit Care Med 194: 1483-1493.
- Revill SM, Morgan MD, Singh SJ, Williams J, Hardman AE (1999) The endurance shuttle walk: a new field test for the assessment of endurance capacity in chronic obstructive pulmonary disease. Thorax 54: 213-222.
- Casanova C, Cote CG, Marin JM, de Torres JP, Aguirre-Jaime A, et al. (2007) The 6-min walking distance: long-term follow up in patients with COPD. Eur Respir J 29: 535-540.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes M,et al. (2004) The body-mass index airflow obstruction dyspnea and exercise capacity index in chronic obstructive pulmonary disease. New Eng J Med 350: 1005-1012.
- Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, et al. (1994) Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 272: 1497-1505.
- Pauwels RA, Lofdahl CG, Laitinen LA, Schouten JP, Postma DS, et al. (1999) Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European respiratory society study on chronic obstructive pulmonary disease. N Engl J Med 340: 1948-1953.
- Vestbo J, SorensenT, Lange P, Brix A, Torre P, et al. (1999) Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 353: 1819-1823.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, et al. (2008) A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 359: 1543-1554.
- Farne HA, Cates CJ (2015) Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 22: CD008989.
- van der Molen T, Cazzola M (2012) Beyond lung function in COPD management: effectiveness of LABA/LAMA combination therapy on patient-centred outcomes. Prim Care Respir J. 21: 101-108.
- Mahler DA, Decramer M, D'Urzo A, Worth H, White T, et al. (2014) Dual bronchodilation with QVA149 reduces patient-reported dyspnoea in COPD: The BLAZE study. Eur Respir J 43: 1599-1609.
- Wedzicha JA, Decramer M, Ficker JH, Nierwoehner DE, Sandström T, et al. (2013) Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): A randomised double-blind parallel-group study. Lancet Respir Med 1: 199-209.
- Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, et al. (2016) Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med 374: 2222-2234.
- Kew KM, Mavergames C, Walters JA (2013) Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 10: CD010177.
- McGarvey L, Niewoehner D, Magder S, Sachs P, Tetzlaff K, et al. (2015) One-year safety of olodaterol once daily via respimat® in patients with GOLD 2-4 chronic obstructive pulmonary disease: Results of a pre-specified pooled analysis. COPD 12: 484-493.
- Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, et al. (2010) Efficacy of a new once-daily long-acting inhaled beta2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax 65: 473-479.
- Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten-van Mölken MP, et al. (2011) Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med 364: 1093-1103.
- Decramer ML, Chapman KR, Dahl R, Frith P, Devouassoux G, et al. (2013) Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): A randomised blinded parallel-group study. Lancet Respir Med 1: 524-533.
- Michele TM, Pinheiro S, Iyasu S (2010) The safety of tiotropium--the FDA's conclusions. N Engl J Med 363: 1097-1099.
- Nannini LJ, Lasserson TJ, Poole P (2012) Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 9: CD006829.
- Nannini LJ, Poole P, MilanSJ, Kesterton A (2013) Combined corticosteroid and long-acting beta[2]-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 8: CD006826.
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, et al. (2007) Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 356: 775-789.
- Vestbo J, Anderson JA, Brook RD, Calverley PM, Celli BR, et al. (2016) Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): A double-blind randomised controlled trial. Lancet 387: 1817-1826.
- Pavord ID, Lettis S, Locantore N, Pascoe S, Jones PW, et al. (2016) Blood eosinophils and inhaled corticosteroid/long-acting beta-2 agonist efficacy in COPD. Thorax 71: 118-125.
- Watz H, Tetzlaff K, Wouters EF, Kirsten A, Magnussen H, et al. (2016) Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med 4: 390-398.
- Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, et al. (2009) Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 374: 685-694.
- Chong J, Leung B, Poole P, Black PN (2013) Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 11: CD002309
- Herath SC, Poole P (2013) Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev 11: CD009764.
- Ni W, Shao X, Cai X, Wei C, Cui J, et al. (2015) Prophylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: a meta-analysis. PloS One.10: e0121257.
- Poole P, Chong J, Cates CJ (2015) Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev 7: CD001287.ÃÆâÃâââ¬Ãâè
- Sandhaus RA, Turino G, Brantly ML, Campos M, Cross CE, et al. (2016) The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. J COPD F 3: 668-682.
- Anthonisen NR, Connett JE, Murray RP (2002) Smoking and lung function of lung health study participants after 11 years. Am J Respir Crit Care Med 166: 675-679.
- Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, et al. (2013) Physician advice for smoking cessation. Cochrane Database Syst Rev 5: CD000165.ÃÆâÃâââ¬Ãâè
- Kottke TE, Battista RN, DeFriese GH, Brekke ML (1988) Attributes of successful smoking cessation interventions in medical practice. A meta-analysis of 39 controlled trials. JAMA 259: 2883-2889.ÃÆâÃâââ¬Ãâè
- Stead LF, Koilpillai P, Fanshawe TR, Lancaster T (2012) Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev 3: CD008286.ÃÆâÃâââ¬Ãâè
- Poole PJ, Chacko E, Wood-Baker RW, Cates CJ (2006) Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 1: CD002733.
- Huang CL, Nguyen PA, Kuo PL, Iqbal U, Hsu YH, et al. (2013) Influenza vaccination and reduction in risk of ischemic heart disease among chronic obstructive pulmonary elderly. Comput Methods Programs Biomed 111: 507-511.
- https://www.cdc.gov/vaccines/vpd/pneumo/index.html
- Walters JA, Smith S, Poole P, Granger RH, Wood-Baker R (2010) Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 11: CD001390.
- Vogiatzis I, Rochester CL, Spruit MA, Troosters T, Clini EM, et al (2016) Increasing implementation and delivery of pulmonary rehabilitation: Key messages from the new ATS/ERS policy statement. Eur Respir J 47: 1336-1341.
- Garvey C, Bayles MP, Hamm LF, Hill K, Holland A, et al. (2016) Pulmonary rehabilitation exercise prescription in chronic obstructive pulmonary disease: review of selected guidelines: An official statement from the american association of cardiovascular and pulmonary rehabilitation. J Cardiopulm Rehail Prev 36: 75-83.
- McCarthy B, Casey D, Devane D, Murphy K, Murphy E, et al. (2015) Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2: CD003793.
- Sahin H, Naz I, Varol Y, Aksel N, Tuksavul F, et al. (2016) Is a pulmonary rehabilitation program effective in COPD patients with chronic hypercapnic failure? Expert Rev Respir Med 10: 593-598.
- Zwerink M, Brusse-Keizer M, van der Valk PD, Zierlhuis GA, Monninkhof EM, et al. (2014) Self-management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 3: CD002990.
- Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, et al. (2003) A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 348: 2059-2073.
- Marchetti N, Criner GJ (2015) Surgical approaches to treating emphysema: Lung volume reduction surgery bullectomy and lung transplantation. Semin Respir Crit Care Med 36: 592-608.
- Deslée G, Mal H, Dutau H, Bourdin A, Vergnon JM, et al. (2016) Lung volume reduction coil treatment vs usual care in patients with severe emphysema: The REVOLENS randomized clinical trial. JAMA 315: 175-184.
- Sciurba FC, Criner GJ, Strange C, Shah PL, Michaud G, et al. (2016) Effect of endobronchial coils vs usual care on exercise tolerance in patients with severe emphysema: The RENEW randomized clinical trial. JAMA 315: 2178-2189.
- Christie JD Edwards LB Kucheryavaya AY Benden C Dipchand AI et al. (2012) The registry of the international society for heart and lung transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant 31: 1073-86.
- Stavem K, Bjortuft O, Borgan O, Geiran O, Boe J (2006) Lung transplantation in patients with chronic obstructive pulmonary disease in a national cohort is without obvious survival benefit. J Heart Lung Transplant 25: 75-84.
- (2004) National consensus project for quality palliative care: Clinical practice guidelines for quality palliative care executive summary. J Palliat Med 7: 611-627.
- Ekstrom M, Nilsson F, Abernethy AA, Currow DC (2015) Effects of opioids on breathlessness and exercise capacity in chronic obstructive pulmonary disease. A systematic review. Ann Am Thorac Soc 12: 1079-1092.
- Rocker GM, Simpson AC, Joanne Young BHSc, Horton R, Sunuff T, et al. (2013) Opioid therapy for refractory dyspnea in patients with advanced chronic obstructive pulmonary disease: Patients’ experiences and outcomes. CMAJ Open 1: E27-36.
- Marciniuk DD, Goodridge D, Hernandez P, Rocker G, Balter M, et al. (2011) Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: A canadian thoracic society clinical practice guideline. Can Respir J 18: 69-78.
- Vieira PJ, Chiappa AM, Cipriano G Jr, Umpierre D, Arena R, et al. (2014) Neuromuscular electrical stimulation improves clinical and physiological function in COPD patients. Respir Med 108: 609-620.
- Galbraith S, Fagan P, Perkins P, Lynch A, Booth S (2010) Does the use of a handheld fan improve chronic dyspnea? A randomized controlled crossover trial. J Pain Symptom Manage 39: 831-838.
- Marchetti N, Lammi MR, Travaline JM, Ciccolella D, Civic B, et al. (2015) Air current applied to the face improves exercise performance in patients with COPD. Lung 193: 725-731.
- Uronis HE, Ekstrom MP, Currow DC, McCrory DC, Samsa GP, et al. (2015) Oxygen for relief of dyspnoea in people with chronic obstructive pulmonary disease who would not qualify for home oxygen: A systematic review and meta-analysis. Thorax 70: 492-494.
- Farver-Vestergaard I, Jacobsen D, Zachariae R (2015) Efficacy of psychosocial interventions on psychological and physical health outcomes in chronic obstructive pulmonary disease: A systematic review and meta-analysis. Psychother Psychosom 84: 37-50.
- Ferreira IM, Brooks D, White J, Goldstein R (2012) Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 12: CD000998.
- Payne C, Wiffen PJ, Martin S (2012) Interventions for fatigue and weight loss in adults with advanced progressive illness. Cochrane Database Syst Rev 1: CD008427.
- Ek K, Andershed B, Sahlberg-Blom E, Ternestedt BM (2015) "The unpredictable death"-the last year of life for patients with advanced COPD: Relatives' stories. Palliat Support Care. 13: 1213-1222.
- Weber C, Stirnemann J, Herrmann FR, Pautex S, Janssens JP (2014) Can early introduction of specialized palliative care limit intensive care emergency and hospital admissions in patients with severe and very severe COPD? a randomized study. BMC Palliat Care 13: 47.
- Cranston JM, Crockett AJ, Moss JR, Alpers JH (2005) Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 4: CD001744.
- Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR (2010) Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med 182: 325-331.
- Souza ML, Meneghini AC, Ferraz E, Vianna EO, Borges MC (2009) Knowledge of and technique for using inhalation devices among asthma patients and COPD patients. J Bras Pneumol 35: 824-331.
- Melani AS, Bonavia M, Cilenti V, Cinti C, Lodi M, et al. (2011) Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med 105: 930-938.
- Sanchis J, Gich I, Pedersen S, Aerosol Drug Management Improvement Team (ADMIT) (2016) Systematic review of errors in inhaler use: Has patient technique improved over time? Chest 150: 394-406.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences