Breathing Intolerance Index (BIT) and its Relation to Exercise Data: Noninvasive Assessment of Inspiratory Muscle Endurance during Rest and Exercise in Patients with Chronic Obstructive Pulmonary Disease and Cardiovascular Disorders
Ahmet Baydur, Amy Tran, Leejoe Pallickal, Michael Fong, Luanda Grazette, Shadman Chowdhury, Zhanghua Chen
DOI10.21767/2572-5548.100011
Ahmet Baydur1*, Amy Tran2, Leejoe Pallickal1, Michael Fong3, Luanda Grazette3, Shadman Chowdhury1 and Zhanghua Chen4
1Divisions of Pulmonary, Critical Care and Sleep Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA
2General and Geriatric Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA
3Cardiovascular Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA
4Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA
- *Corresponding Author:
- Baydur A
Divisions of Pulmonary, Critical Care and Sleep Medicine
Keck School of Medicine, University of Southern California, Los Angeles, CA
Tel: 323-226-7923
Fax: 323-226-2738
E-mail: baydur@usc.edu
Received Date: March 10, 2016; Accepted Date: April 01, 2016; Published Date: April 07, 2016
Citation: Baydur A, Tran A, Pallickal L, et al. (2016) Breathing Intolerance Index (BIT) and its Relation to Exercise Data:Noninvasive Assessment of Inspiratory Muscle Endurance during Rest and Exercise in Patients with Chronic Obstructive Pulmonary Disease and Cardiovascular Disorders. Chron Obstruct Pulmon Dis 1:11. doi: 10.21767/2572-5548.100011
Copyright: © 2016 Baydur A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Rationale: Diaphragmatic tension-time index [TTdi = (Pdi/Pdimax)(Ti/ Ttot)] assesses inspiratory muscle endurance during rest and exercise. However, the procedure is invasive and not practical for general use. Recently, Tidal Volume/Vital Capacity (Vt/VC) has been substituted for Pdi/Pdimax, to assess need for assisted ventilation in chronic respiratory disorders. This technique has not been used to assess respiratory muscle endurance in other conditions during rest and exercise.
Objective: To compare control of ventilation and BIT in cardiorespiratory disorders and controls and to assess their relation to oxygen uptake (V’O2) and annual acute decompensations.
Methods: BIT and V’O2 were assessed in patients with stable CVD (n = 20), RD (n = 20) and C (n = 20) during bicycle ergometry. ANOVA assessed differences in variables amongst cohorts at rest and peak exercise.
Results: Resting BIT was [median (25th, 75th percentiles)] 0.06 (0.04, 0.07), 0.06 (0.04, 0.09) and 0.04 (0.03, 0.05) for CVD, COPD and controls, respectively. Compared to resting, BIT increased by 3.0- to 4.6- fold at peak exercise in all cohorts (p < 0.0001). V’O2max exceeded resting values by 7.5-, 4.6-, and 9.4- fold in COPD, CVD and C, respectively (all p < 0.0001). When corrected for BIT (V’O2/BIT), controls exhibited significantly greater (V’O2/BIT) max than CVD and COPD (p < 0.0001). Amongst patients who acutely decompensated, BIT was higher only in resting CVD patients (p < 0.019).
Conclusions: BIT is useful for evaluating respiratory muscle effort during rest and exercise in CVD and COPD. Oxygen uptake fails to increase in proportion to BIT in these conditions as much as in controls, reflecting impaired oxygen utilization. Its potential usefulness in predicting acute decompensations should be assessed in larger prospective studies.
Keywords
Breathing intolerance index; Exercise; Cardiomyopathies; Chronic obstructive pulmonary disease; Control of ventilation
Abbreviations
AECOPD: Acute Exacerbation Of COPD; ANOVA: Analysis Of Variance; BIT index: Breathing Intolerance Index; FEV1: Forced Expiratory Volume In One Second; FRC: Functional Residual Capacity; FVC: Forced Vital Capacity; IC: Inspiratory Capacity; P0.1: Occlusion Pressure in 0.1 Second; RV: Residual Volume; Vt: Tidal volume; tEFL: Tidal Expiratory Flow Limitation; Ti: Inspiratory Time; Te: Expiratory Time; TTdi: Tension-Time Index Of The Diaphragm; TTmus: Tension-Time Index Of Inspiratory Muscles; V’E: Minute Ventilation; V’O2: Oxygen Uptake
Introduction
Patients with respiratory disorders experience ventilatory impairment related to underlying changes in lung volume, respiratory compliance and resistance, and breathing control mechanisms. These factors affect the diaphragm’s ability to sustain ventilation in the face of imposed respiratory loads. Tension time index of the diaphragm [TTdi = product of the ratio of the mean transdiaphragmatic pressure swing divided by the maximum transdiaphragmatic pressure (Pdi/Pdimax) with the inspiratory time divided by the total breath time (Ti/ Ttot)] is related to diaphragm endurance [1].
The ability to sustain transdiaphragmatic pressure swings required for continuous spontaneous breathing cannot be sustained for more than 45 minutes when the TTIdi is >0.15. However, the method of Bellemare and Grassino [1] is invasive, requiring the use of gastric and esophageal balloons, and is difficult for untrained subjects to perform.
Later, Ramonatxo et al. [2] instituted a noninvasive method for assessing the tension-time index (based on mouth occlusion pressure, P 0.1) and easy to use from a technical standpoint. Koga et al. [3] substituted the ratio of tidal volume to vital capacity (Vt/VC) for Pdi/Pdimax in the Bellemare and Grassino [1] relationship, calling this relationship [(Ti/Ttot) x (Vt/FVC)] the breathing intolerance index (BIT). They found that the BIT indices of patients with respiratory failure requiring noninvasive assisted ventilation (NIV) were higher than those who did not need NIV. Vt/VC can substitute for Pdi/ Pdimax because the more Vt approaches VC, the less ability the inspiratory muscles have to sustain alveolar ventilation [1].
The study of Koga et al. [3], however, assessed the BIT index in quietly breathing patients mainly with bronchial asthma and restrictive thoracic/neuromuscular disorders. Baydur and Chen [4] measured BIT in patients with obesity and COPD during resting breathing in seated and supine postures, finding that BIT was significantly greater in seated position in both cohorts due primarily to an increase in Vt in this position. BIT tended to be higher in patients with obesity and COPD as compared to control subjects.
In patients with cardiovascular disease, most notably those with chronic heart failure, respiratory muscle weakness has been documented by diminished maximal inspiratory pressures [5-7]. The tension-time index of the diaphragm is markedly increased in patients with chronic heart failure in contrast to healthy subjects [8], approaching levels shown to generate fatigue, as demonstrated by Bellemare and Grassino [1].
BIT has not been assessed in patients with cardiovascular and other respiratory disorders at rest or during exercise. Its measurement would be useful in determining the threshold for respiratory muscle fatigue and degree of respiratory impairment in such disorders.
The purpose of this study was to (1) compare control of ventilation and BIT during rest and peak exercise in individuals with cardiovascular and ventilatory limitation (due to chronic obstructive pulmonary disease, COPD) and control subjects free of cardiorespiratory illness, (2) to assess the relationship of BIT to oxygen uptake at rest and peak exercise, and (3) to assess the relationship of BIT to the likelihood of developing acute exacerbations of COPD (AECOPD) and acute heart failure.
Methods
Subjects
The records of clinically stable subjects with chronic heart failure (all cardiomyopathies, 13 ischemic and 7 dilated, non ischemic) and COPD referred for cardiopulmonary exercise testing were reviewed. Patients underwent testing for evaluation of exercise tolerance and response to medical therapy. All cardiomyopathy patients were in a clinically stable condition with no worsening of heart failure or change of cardiac medications in the previous 2 months.
All patients were receiving angiotensin converting enzyme inhibitors or angiotensin receptor antagonists and betablocking agents; some patients were also receiving diuretics, and a few, calcium-channel blockers.
Severity of cardiac dysfunction was classified as New York Heart Association Severity II and III; all patients had a resting left ventricular ejection fraction of 50% or less as assessed by echocardiography. The diagnosis of COPD was based on GOLD/ European Respiratory Society recommendations [9].
History concerning medical and smoking history, and respiratory symptoms, as well as hospitalizations for acute cardiorespiratory illness were recorded. Patients with asthma, primary restrictive respiratory disorders and acute cardiorespiratory illnesses were excluded. Control subjects were individuals free of cardiorespiratory illness.
The study was approved by the Institutional Review Board (HS-14-00244). Written consent was obtained from all subjects as required by the Board.
Pulmonary function testing
Spirometry, lung volumes by body plethysmography and gas transfer measurements were performed while seated with a Collins GS/PLUS or DSII/PLUS system (Warren Collins; Braintree, MA, or Ultima PF, MedGraphics, Saint Paul, MN). The cut-off point of FEV1/FVC for COPD was 0.7 [9]. Predicted values for post-bronchodilator FEV1, FVC and FEV1/FVC were from Schoenberg et al. [10], and for subdivisions of lung volume, from Crapo et al. [11].
Exercise testing and control of ventilation
Subjects refrained from eating or drinking coffee for at least 12 hours before testing. They breathed room air through the equipment assembly with a nose clip on. The exercise test was performed on a calibrated bicycle ergometer (Lode, Amsterdam, Netherlands).
During the test, subjects wore a noseclip and breathed through a low resistance (1.5 cm H2O/L/s) and low dead space (45 mL) breathing valve. The valve was connected by the expiratory circuit to a breath-by-breath automated exercise metabolic system (Ultima CardiO2, MedGraphics, St. Paul, Minnesota).
Flow was measured with a heated bidirectional Pitot tube flow sensor and differential pressure transducer (MedGraphics) that was linear over the experimental range of flow up to 14 L/s. Volume was obtained by integration of digitized flow. A closed system ensured that end-expiratory volume remained constant. Each subject underwent a 3 min trial run in order to become accustomed to the procedure.
Subjects were monitored for leaks at the mouthpiece. The system continuously measured oxygen uptake, carbon dioxide output, and respiratory exchange ratio. Before each test, the gas analyzers were calibrated with two gas mixtures of known oxygen and carbon dioxide concentration. Heart rate was continuously recorded on a cardioscope and electrocardiogram periodically recorded.
The subjects then performed an incremental exercise test on the ergometer in seated position. Resting measurements were obtained over 5 minutes at rest on the cycle. Following the warm-up period the workload was increased by 10 Watts every 90 sec for patients with COPD and CVD and by 20 Watts every 90 sec for the control subjects. All subjects were encouraged to perform up to their maximal abilities until they felt unable to continue or they reached their maximal oxygen uptake (V’O2).
Patients ended their exercise because of dyspnea, leg fatigue, or both. For the control subjects, indications that they had reached V’O2max included stability of heart rate close to their predicted maximal heart rate, stability of V’O2 despite increase in the work load, achieving a respiratory exchange ratio of at least 1.1, and inability of the subject to maintain a pedaling rate of 50 rpm.
Data acquisition began after regular breathing was achieved and was continued for at least 5 minute post-exercise for purposes of expired gas analysis and ECG monitoring.The data were averaged during the last 20 sec of each load over an integral number of breaths during which steady state had been achieved.
Tidal volume (Vt), inspiratory time (Ti), and total time of the respiratory cycle (Ttot) were measured. From these values, the duty cycle (Ti/Ttot) and mean inspiratory flow (Vt/Ti) which corresponds to an intensity index of inspiratory activity (index of neural drive) were computed [12,13].
BIT was computed as (Ti/Ttot) × (Vt/FVC) [3], where Vt and Ti/Ttot were determined during steady states at the end of the pre-exercise and peak exercise periods, respectively. Coefficients of variation for tidal volume (Vt), inspiratory time (Ti), and expiratory time (V’E) were 5%-15%, as reported for normal subjects and patients with COPD [12-15].
Data analysis
Characteristics of participants compared across cardiovascular, COPD and control cohorts using linear regression, where each characteristic variable was inverse cumulative normal transformed [16]. Age, sex and BMI were adjusted for in the model for their potential confounding effect. Kruskal-Wallis test was used to compare the exacerbations between COPD and cardiovascular groups [17].
Notched boxplots were used to present the distribution of lung function variables across groups at rest or peak exercise. The notches surrounding the medians provide a measure of the rough significance of differences between the values. Specifically, if the notches about two medians do not overlap in this display, the medians are significantly different at about a 95% confidence level (that is, p < 0.05) [18].
For each of the variables investigated, linear regressions that inverse cumulative normal transformed variable as a function of the indicator of cohorts and covariates including age, sex and BMI was modeled to assess differences in central tendency between subjects with cardiovascular, COPD and controls [16].
The Wilcoxon signed rank test was used to test for differences between variables obtained at rest and peak exercise. Relationships between V’O2 (resting and max) and BIT index (expressed as V’O2/BIT), and between BIT index and numbers of acute exacerbations of COPD (AECOPD) and episodes of heart failure per annum were assessed. The BIT index was further analyzed by plotting isopleths of Vt/VC versus Ti/Ttot, and of BIT vs V’O2, on which median values of BIT for each cohort were placed [1,3].
Results
Anthropometric and spirometric data
Of 77 patients screened, excluded from analysis were 9 patients with restrictive lung disease, and 8 with idiopathic pulmonary hypertension. The remaining 60 subjects consisted of 20 patients each with COPD, cardiovascular disease (CVD) (New York Heart Association classifications II and III) and nonsmoking healthy subjects (C) (Table 1).
Ten (50%) of the cardiomyopathy patients were past smokers; none were currently smoking. Sixteen (80%) COPD patients were past smokers and 4 (5%) were still smoking. The median ages of the CVD and COPD patients were greater than the control subjects, and median BMI was slightly higher in the CVD group, but these differences were statistically insignificant.
Compared to the control group, both FEV1 and FVC were lower in the CVD and COPD cohorts (p < 0.0001), while FEV1/FVC in COPD patients was significantly lower than in the other two cohorts (p < 0.0001).
Control of ventilation and BIT index
Figures 1-4 show that Vt and Vt/Ti in the control group at peak exercise were significantly higher than in the CVD and COPD cohorts (all p ≤ 0.0003), but did not differ amongst the cohorts at rest.
Figure 1: Vt data for patients with, COPD, and control subjects.
Tidal volume (Vt) at rest and peak exercise in the three cohorts. See text for details. Each notched box represents the 25th-75th percentile; internal line indicates the median; whiskers indicate the 10th and 90th percentiles. Notches extend to +/-1.58 inter-quartile range/ n1/2. Notches that do not overlap suggest strong evidence of different medians.
The general linear model used to model the normal ranks of the test variable as a function of the resting and peak exercise groups, adjusted for age, sex and BMI, showed no significant differences amongst cohorts at rest, p = 0.10, but did show differences amongst cohorts at peak exercise, p < 0.0001.
P-values presented in the figure were obtained from Wilcoxon signed rank test (non-parametric paired-sample t-test) for comparisons between rest and peak exercise in each cohort.
COPD: chronic obstructive pulmonary disease; CVD: cardiovascular disease
Figure 2: Ti data for patients with COPD, and control subjects.
Inspiratory time (Ti) at rest and peak exercise in the three cohorts. Each notched box represents the 25th-75th percentile;
internal line indicates the median; whiskers indicate the 10th and 90th percentiles. Notches extend to +/-1.58 inter-quartile range/ n1/2. Notches that do not overlap suggest strong evidence of different medians.
The general linear model used to model the normal ranks of the test variable as a function of the resting and peak exercise groups, adjusted for age, sex and BMI, showed no significant differences amongst cohorts at rest, p = 0.92, or peak exercise, p = 0.06. See text for details.
P-values presented in the figure were obtained from Wilcoxon signed rank test (non-parametric paired-sample t-test) for comparisons between rest and peak exercise in each cohort.
COPD: chronic obstructive pulmonary disease; CVD: cardiovascular disease
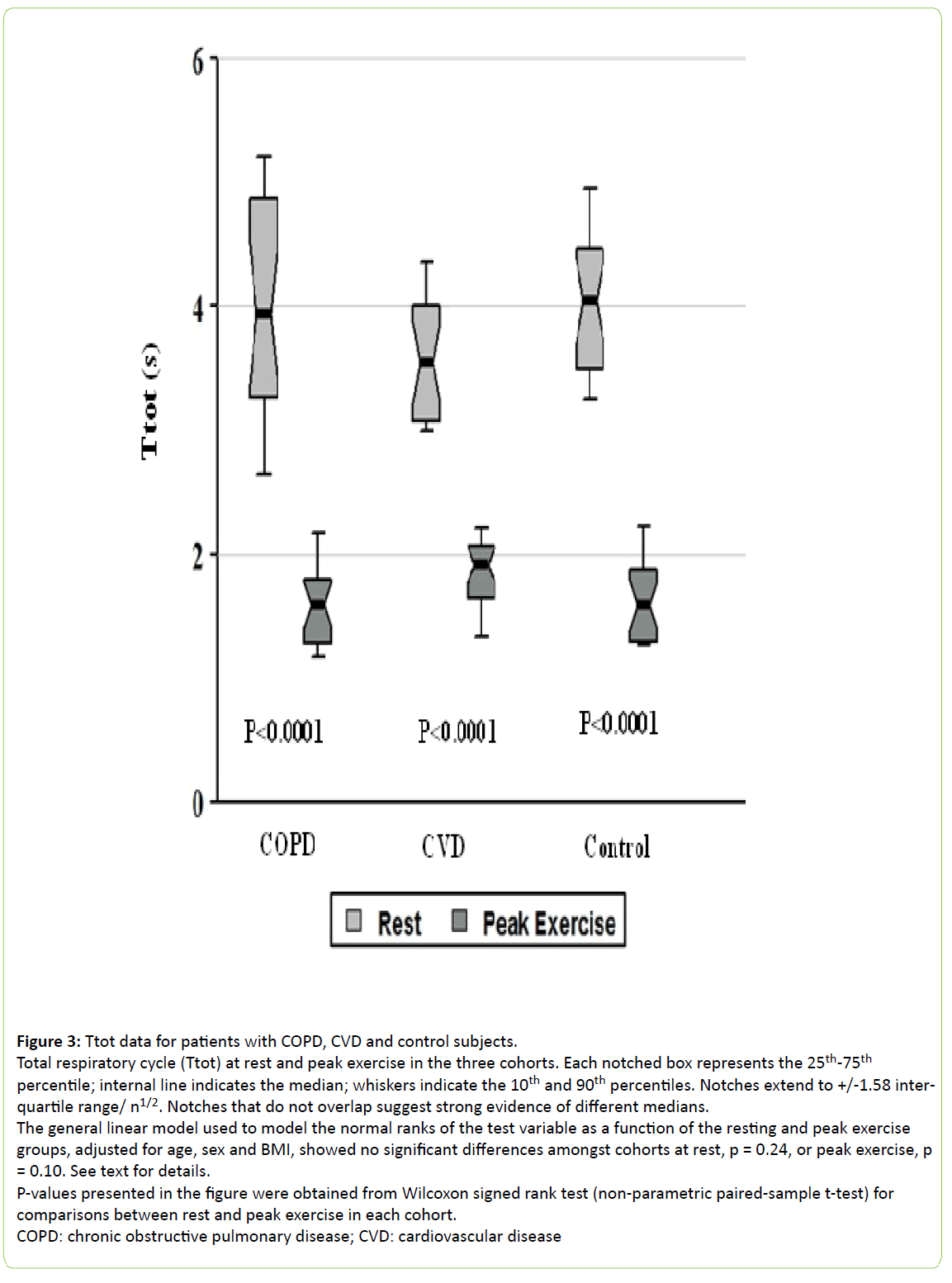
Figure 3: Ttot data for patients with COPD, CVD and control subjects.
Total respiratory cycle (Ttot) at rest and peak exercise in the three cohorts. Each notched box represents the 25th-75th percentile; internal line indicates the median; whiskers indicate the 10th and 90th percentiles. Notches extend to +/-1.58 interquartile range/ n1/2. Notches that do not overlap suggest strong evidence of different medians.
The general linear model used to model the normal ranks of the test variable as a function of the resting and peak exercise groups, adjusted for age, sex and BMI, showed no significant differences amongst cohorts at rest, p = 0.24, or peak exercise, p = 0.10. See text for details.
P-values presented in the figure were obtained from Wilcoxon signed rank test (non-parametric paired-sample t-test) for comparisons between rest and peak exercise in each cohort.
COPD: chronic obstructive pulmonary disease; CVD: cardiovascular disease
Figure 4: Vt/Ti data for patients with COPD, CVD and control subjects.
Mean inspiratory flow (Vt/Ti) at rest and peak exercise in the three cohorts. Each notched box represents the 25th-75th percentile; internal line indicates the median; whiskers indicate the 10th and 90th percentiles. Notches extend to +/-1.58 interquartile range/ n1/2. Notches that do not overlap suggest strong evidence of different medians.
The general linear model used to model the normal ranks of the test variable as a function of the resting and peak exercise groups, adjusted for age, sex and BMI, showed no significant differences amongst cohorts at rest, p = 0.77, but did show differences amongst cohorts at peak exercise, p = 0.0003. See text for details.
P-values presented in the figure were obtained from Wilcoxon signed rank test (non-parametric paired-sample t-test) for comparisons between rest and peak exercise in each cohort.
COPD: chronic obstructive pulmonary disease; CVD: cardiovascular disease
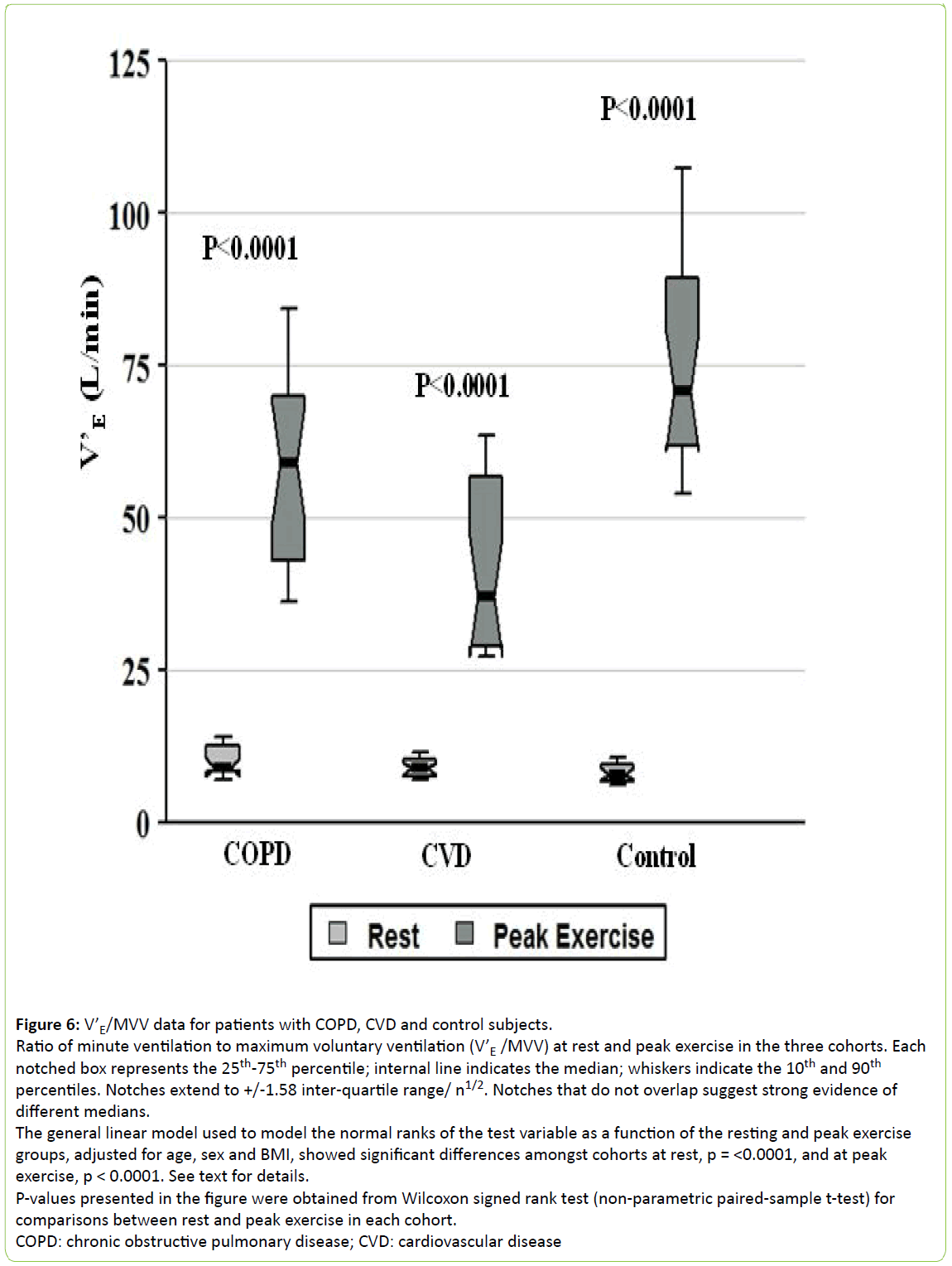
The CVD cohort exhibited the lowest values for Vt/Ti, minute ventilation (V’E) and V’E/MVV at peak exercise (all p < 0.0003, Figures 4-6, respectively), while the COPD patients showed the highest V’E/MVV (i.e., the lowest ventilatory reserve) at peak exercise (Figure 6).
Figure 5: Minute ventilation (V’E) data for patients with COPD, CVD and control subjects.
Minute ventilation (V’E) at rest and peak exercise in the three cohorts. Each notched box represents the 25th-75th percentile; internal line indicates the median; whiskers indicate the 10th and 90th percentiles. Notches extend to +/-1.58 inter-quartile range/ n1/2. Notches that do not overlap suggest strong evidence of different medians.
The general linear model used to model the normal ranks of the test variable as a function of the resting and peak exercise groups, adjusted for age, sex and BMI, showed no significant differences amongst cohorts at rest, p = 0.18, but did show differences amongst cohorts at peak exercise, p < 0.0001. See text for details.
P-values presented in the figure were obtained from Wilcoxon signed rank test (non-parametric paired-sample t-test) for comparisons between rest and peak exercise in each cohort.
COPD: chronic obstructive pulmonary disease; CVD: cardiovascular disease
Figure 6: V’E/MVV data for patients with COPD, CVD and control subjects.
Ratio of minute ventilation to maximum voluntary ventilation (V’E /MVV) at rest and peak exercise in the three cohorts. Each notched box represents the 25th-75th percentile; internal line indicates the median; whiskers indicate the 10th and 90th percentiles. Notches extend to +/-1.58 inter-quartile range/ n1/2. Notches that do not overlap suggest strong evidence of different medians.
The general linear model used to model the normal ranks of the test variable as a function of the resting and peak exercise
groups, adjusted for age, sex and BMI, showed significant differences amongst cohorts at rest, p = <0.0001, and at peak
exercise, p < 0.0001. See text for details.
P-values presented in the figure were obtained from Wilcoxon signed rank test (non-parametric paired-sample t-test) for
comparisons between rest and peak exercise in each cohort.
COPD: chronic obstructive pulmonary disease; CVD: cardiovascular disease
Duty cycle, Ti/Tot, significantly increased during exercise, but did not differ amongst cohorts either at rest or during peak exercise (Figure 7).
Figure 7: Ti/Ttot data for patients with COPD, CVD and control subjects.
Duty cycle (Ti/Ttot) at rest and peak exercise in the three cohorts. Each notched box represents the 25th-75th percentile; internal line indicates the median; whiskers indicate the 10th and 90th percentiles. Notches extend to +/-1.58 inter-quartile range/ n1/2. Notches that do not overlap suggest strong evidence of different medians.
The general linear model used to model the normal ranks of the test variable as a function of the resting and peak exercise groups, adjusted for age, sex and BMI, showed no significant differences amongst cohorts at rest, p = 0.66, or peak exercise, p = 0.92. See text for details.
P-values presented in the figure were obtained from Wilcoxon signed rank test (non-parametric paired-sample t-test) for comparisons between rest and peak exercise in each cohort.
COPD: chronic obstructive pulmonary disease; CVD: cardiovascular disease
Compared to resting, BIT increased by 3.1-, 3.0- and 4.6- fold at peak exercise in COPD, CVD and control groups, respectively (all p < 0.0001, Figure 8).
Figure 8: Breathing intolerance (BIT) index for patients with COPD, CVD and control subjects.
Breathing intolerance index (BIT) at rest and peak exercise in the three cohorts. Each notched box represents the 25th-75th percentile; internal line indicates the median; whiskers indicate the 10th and 90th percentiles. Notches extend to +/-1.58 interquartile range/ n1/2. Notches that do not overlap suggest strong evidence of different medians.
The general linear model used to model the normal ranks of the test variable as a function of the resting and peak exercise groups, adjusted for age, sex and BMI, showed significant differences amongst cohorts at rest, p = 0.016, but not at peak exercise, p = 0.72. See text for details.
P-values presented in the figure were obtained from Wilcoxon signed rank test (non-parametric paired-sample t-test) for comparisons between rest and peak exercise in each cohort.
COPD: chronic obstructive pulmonary disease; CVD: cardiovascular disease
It was higher in the COPD and CVD groups only at rest (p < 0.016) because of their lower FVCs, but this difference became insignificant as their tidal volumes increased during exercise.
The ability of the BIT index to assess respiratory muscle impairment at baseline and maximal exercise in each cohort was depicted by iso-BIT index graphs (analogous to the isodiaphragmatic tension-time plot of Bellemare and Grassino [1]) in which Ti/Ttot was plotted against Vt/FVC at rest and peak exercise with BIT isopleths ranging from 0.04 to 0.16 (Figure 9). Vt/FVC for all three cohorts increased at peak exercise (all p < 0.0001). Coupled with increases in the duty cycle at peak exercise (Figure 7), these changes resulted in significant increases in the BIT index, as noted in Figure 8.
Figure 9: Ti/Ttot versus Vt/FVC for patients with COPD, CVD and control subjects.
Ti/Ttot versus Vt/FVC at peak exercise (solid symbols) and rest (hollow symbols).
Diagram is analogous to the iso-diaphragmatic tension-time index plot of Bellemare and Grassino [1] and is constructed from the data of our patients. Ordinate: inspiratory to total cycle duration ratio, or duty cycle (Ti/Ttot); abscissa: mean tidal volume expressed as a fraction of the forced vital capacity (Vt/FVC).
The product of the two variables is the breathing intolerance (BIT) index. Four isopleths are drawn for reference. Each symbol refers to the median (inter-quartile range) of each group.
Exercise data and gas exchange measurements
Median duration of the exercise segment during testing for COPD, CVD and control subjects was 8.1, 7.2, and 12.3 minutes, respectively.
Figures 10-13 show the variables of cardiopulmonary exercise testing. As can be seen, compared to resting values, median V’O2 increased by 7.5-, 4.6- and 9.4- fold at peak exercise in the COPD, CVD and control groups, respectively (all differences significant at p < 0.0001, Figure 10).
Figure 10: Oxygen uptake (V’O2) at rest and peak exercise for patients with COPD, CVD and control subjects.
V’O2 at rest and peak exercise in the three cohorts. Each notched box represents the 25th-75th percentile; internal line indicates the median; whiskers indicate the 10th and 90th percentiles. Notches extend to +/-1.58 inter-quartile range/ n1/2.
Notches that do not overlap suggest strong evidence of different medians.
The general linear model used to model the normal ranks of the test variable as a function of the resting and peak exercise groups, adjusted for age, sex and BMI, showed no significant differences amongst cohorts at rest, p = 0.63, but were significantly different amongst cohorts at peak exercise, p < 0.0001, with the CVD group exhibiting the lowest value. See text for details.
P-values presented in the figure were obtained from Wilcoxon signed rank test (non-parametric paired-sample t-test) for comparisons between rest and peak exercise in each cohort.
COPD: chronic obstructive pulmonary disease; CVD: cardiovascular disease
At rest, the median O2-pulse, an index of stroke volume, did not significantly differ amongst cohorts (Figure 11); however, in the COPD, CVD and control groups it increased by 2.8-, 2.7- and 4.2- fold at peak exercise, respectively (all p < 0.0001).
Figure 11: O2-pulse (V’O2/HR) at rest and peak exercise for patients with COPD, CVD and control subjects.
V’O2/HR at rest and peak exercise in the three cohorts. Each notched box represents the 25th-75th percentile; internal line indicates the median; whiskers indicate the 10th and 90th percentiles. Notches extend to +/-1.58 inter-quartile range/ n1/2.
Notches that do not overlap suggest strong evidence of different medians.
The general linear model used to model the normal ranks of the test variable as a function of the resting and peak exercise groups, adjusted for age, sex and BMI, showed no significant differences amongst cohorts at rest, p = 0.67, but were significantly different amongst cohorts at peak exercise, p = 0.0001. See text for details.
P-values presented in the figure were obtained from Wilcoxon signed rank test (non-parametric paired-sample t-test) for comparisons between rest and peak exercise in each cohort.
COPD: chronic obstructive pulmonary disease; CVD: cardiovascular disease
Notably, the O2 - pulse in the CVD group reached only 76% and 67% of the corresponding COPD and control group values, respectively, at peak exercise (both differences significant at p < 0.0001).
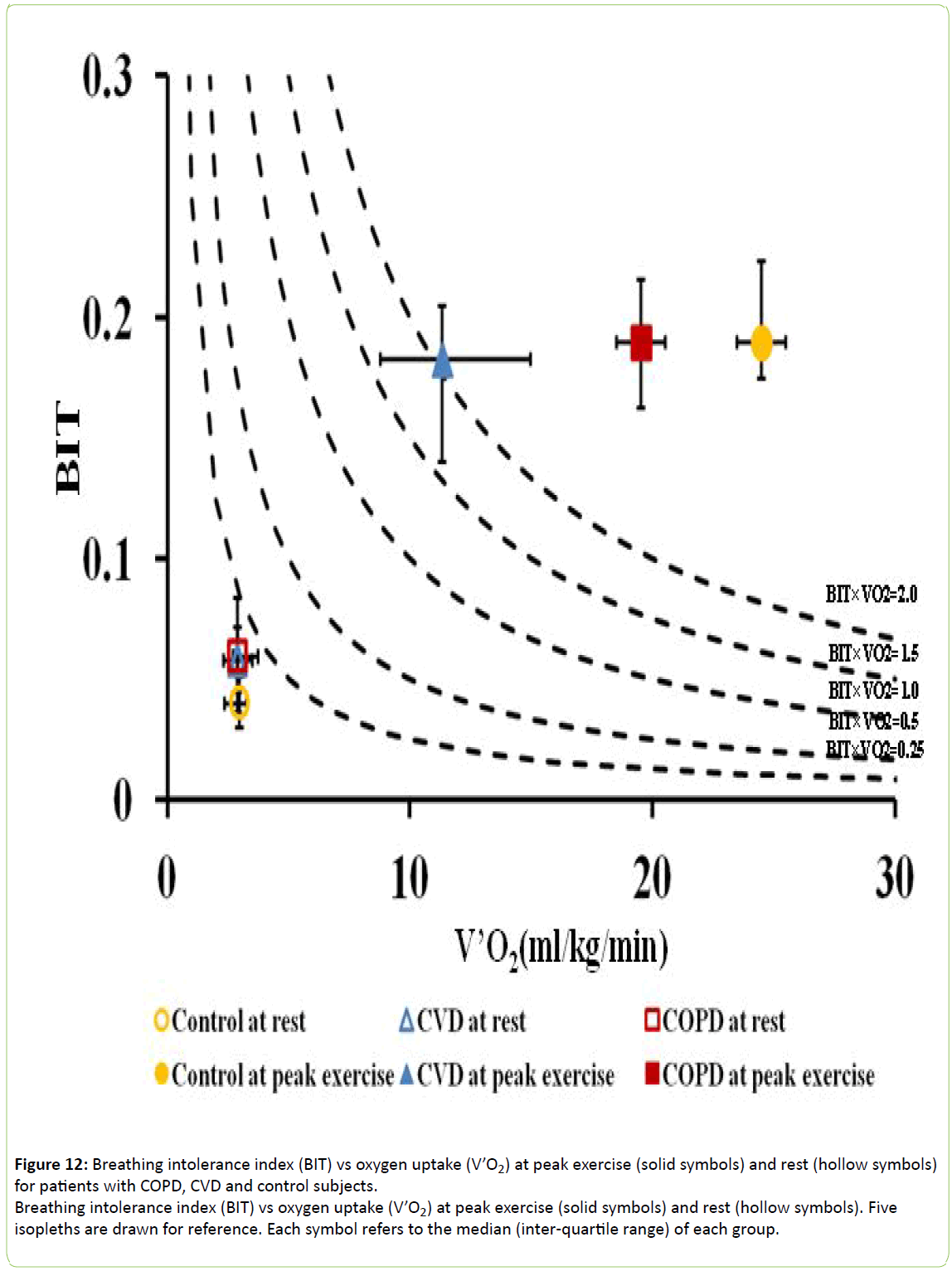
To express V’O2 in relation to BIT, isopleths of its product with BIT were plotted as shown in Figure 12. Control subjects exhibited significantly greater values at peak exercise (without difference in BIT) than the CVD and COPD cohorts (p < 0.0001) indicating their greater oxygen uptake with respect to ventilatory effort.
Figure 12: Breathing intolerance index (BIT) vs oxygen uptake (V’O2) at peak exercise (solid symbols) and rest (hollow symbols) for patients with COPD, CVD and control subjects.
Breathing intolerance index (BIT) vs oxygen uptake (V’O2) at peak exercise (solid symbols) and rest (hollow symbols). Five isopleths are drawn for reference. Each symbol refers to the median (inter-quartile range) of each group.
Episodes of AECOPD and acute heart failure
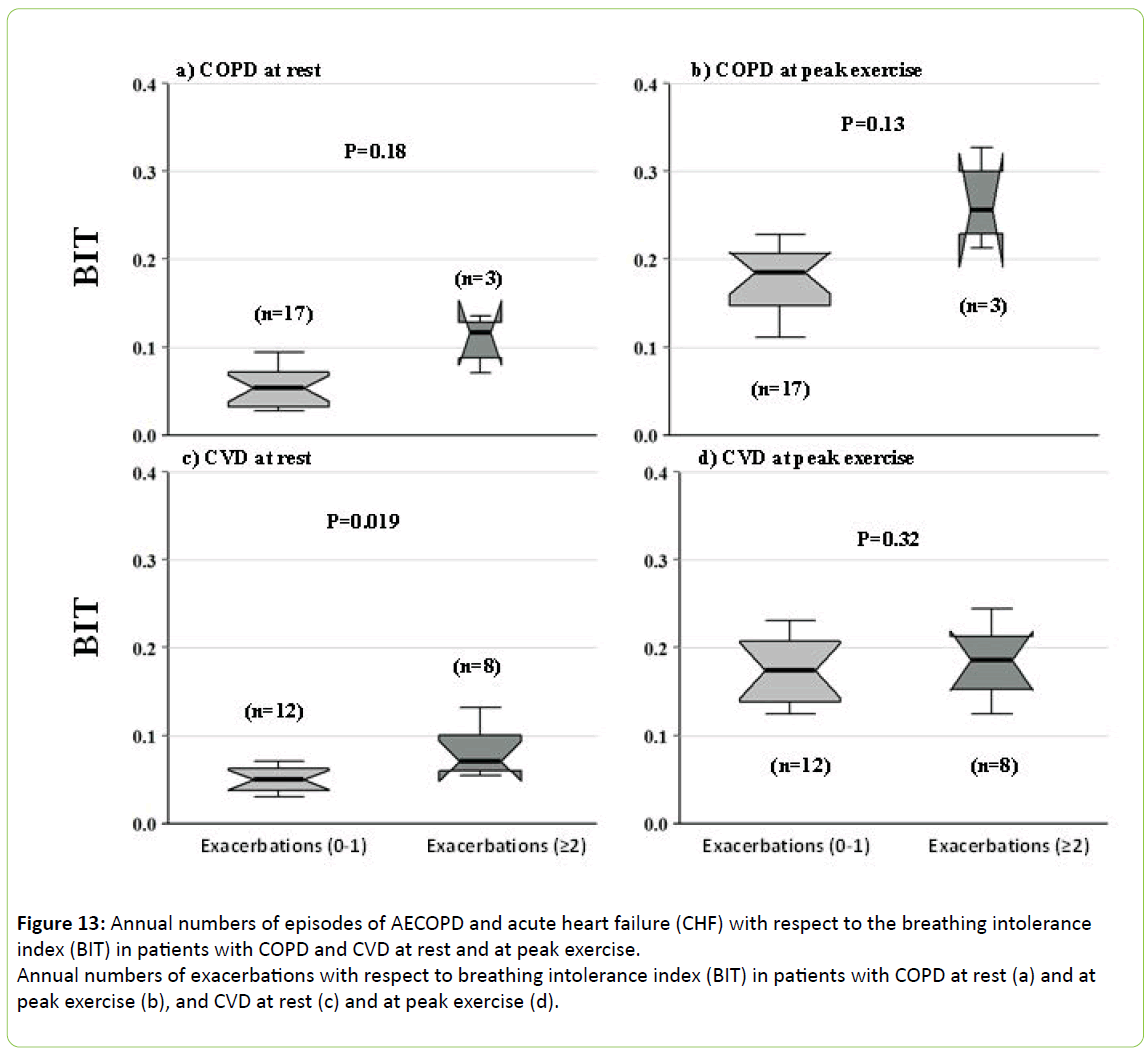
Figure 13 depicts the relation of BIT index at rest and peak exercise to the annual numbers of AECOPD and acute heart failure in COPD and CVD patients, respectively. Resting BIT was 40% higher in the 8 resting CVD patients who experienced acute heart failure (p < 0.019). There were only 3 patients who experienced AECOPD requiring hospitalization, and so while their resting BIT was more than twice as high as in the 17 who did not decompensate, the difference did not reach significance (p < 0.13). No patients required assisted ventilation or died during the period of this study.
Figure 13: Annual numbers of episodes of AECOPD and acute heart failure (CHF) with respect to the breathing intolerance index (BIT) in patients with COPD and CVD at rest and at peak exercise.
Annual numbers of exacerbations with respect to breathing intolerance index (BIT) in patients with COPD at rest (a) and at peak exercise (b), and CVD at rest (c) and at peak exercise (d).
Discussion
To our knowledge this study is the first to evaluate respiratory muscle effort using a noninvasive method comparing patients with CVD and COPD under resting and exercise conditions. The method of assessment was the breathing intolerance index (BIT) that until recently had been used only to evaluate need for assisted ventilation in patients with asthma, chest wall and neuromuscular disorders [1,19]. The key findings in this study are that (a) resting BIT was similar amongst the CVD, COPD and control groups, (b) compared to resting values, BIT increased by several-fold at maximal exercise in all cohorts, and to the greatest degree in the control group, (c) oxygen uptake increased out of proportion to the increase in BIT with exercise in all cohorts, but less so in the CVD and COPD patients, confirming less efficient oxygen uptake for the increase in ventilatory effort at V’O2max in CVD and COPD than in the controls.
Control of ventilation and its relation to BIT in COPD and CVD patients
At rest, the ventilatory pattern (Vt, Ti, Ttot, V’E, Vt/Ti) observed in our COPD and CVD patients was similar to that reported to that by other investigators [4,5,12-15,19-23]. Bellemare and Grassino [1] showed that the critical product of Pdi/Pdimax and Ti/Ttot, tension-time index (TTdi) leading to diaphragmatic fatigue was 0.15 during inspiratory resistive loading at normal resting V’E. In a later study, these authors showed similar results in COPD patients who developed respiratory fatigue by increasing their duty cycle (Ti/Ttot) [20]. Garcia-Rio [24] found that the TTdi in neuromuscular patients (0.145) was about twice as high as in patients with chest wall disorders (0.074) and usual interstitial pneumonia (0.077), and more than twice as high as in control subjects (0.058) because of the higher percentage of the maximal Pdimax generated during tidal breathing. In addition, using mean inspiratory mouth pressure (PI) as a noninvasive means of estimating respiratory muscle effort, these authors found that, compared to control subjects, PI was 65% to 241% higher in their patients with restrictive respiratory disorders. Koga et al. [3] showed that during resting breathing, BIT index was, on average, 0.186 in patients with lung and chest wall disorders requiring nocturnal noninvasive positive-pressure ventilation, significantly greater than in non-ventilator users (0.087) and healthy volunteers (0.05), the last values being similar to those of our cohorts at rest. Similarly, Bach and colleagues [19] showed that in patients requiring noninvasive ventilatory support, BIT was 2- to 3- fold greater during spontaneous breathing as compared to patients who did not need assisted ventilation. Resting BIT indices in our control subjects (median 0.04) was similar to that of nonsmoking healthy individuals reported by Koga et al. (0.05) [3]; in our CVD and COPD patients BIT indices were slightly higher than in controls (0.06) but similar to patients with mild to moderate respiratory impairment not requiring noninvasive ventilation (0.057-0.085) reported by Koga et al. [3]. Thus, as expected, resting BIT indices in our patients were in the range that would not predict the likelihood of AECOPD or acute CHF. Clearly, the ventilatory reserves of our patients, while less than in control subjects, are greater than those of neuromuscular and chest wall patients reported by Koga et al. [3]. During resting breathing, BIT becomes useful as a predictor for acute exacerbation likely shortly before overt respiratory failure, and does so by an increase above resting stable conditions.
Chronic obstructive pulmonary disease
In the present study, no patients required assisted ventilation as expected by absence of significant differences in BIT indices amongst cohorts. At peak exercise, the COPD group encroached on their ventilatory reserve, reaching 76% of their maximum voluntary ventilation, significantly higher than the CVD and control groups. The diagnosis of respiratory fatigue is a challenge because of the lack of universally agreed criteria and technical difficulties [25]. Nevertheless, even though patients with respiratory impairment are able to maintain spontaneous ventilation, they may be in the “fatigue process”, as described by Vassilakopoulos et al. [26]. According to predictions of the TTdi concept, overt fatigue and task failure (that is, not being able to breathe spontaneously) eventually develop over time. Therefore, fatigue should be considered as a continuum, and what determines ability to sustain spontaneous breathing is whether one is in the “fatigue process”. In COPD patients who fail to be weaned off mechanical ventilation, TTdi is 38% higher than in patients who are successfully weaned off ventilation [26], and higher than 0.15, the critical value above which diaphragmatic fatigue may occur in normal subjects [1] and COPD [20]. During exercise, even patients with mild COPD exhibit increased dead space (Vd) ventilation, leading to compensatory increase in minute ventilation to maintain a constant alveolar ventilation [27], resulting in early exercise intolerance. In fact, in the study of Elbehairy [27] the patient group exhibited levels of PaCO2 lower than in the control group throughout exercise, suggesting cortical or behavioral input accounting for the hypocapnia [28].
Cardiovascular disease
Values for BIT at rest in our CVD patients were, on average, 50% higher than in control subjects. These values were slightly lower than the tension-time index of respiratory muscles measured by a different non-invasive technique, in which the product of the ratio of mean inspiratory pressure to maximal inspiratory pressure and the duty cycle [TTdin = (Pi/Pimax) (Ti/ Ttot) = 0.08] was used [22]. In our CVD patients BIT increased three-fold at peak exercise, similar to the 2.7- fold increase in the tension-time index of inspiratory muscles (TTmus) reported by Vibarel et al. [22]. As was the case with TTmus reported by Vibarel et al. [22], the difference in peak exercise BIT between CVD and controls was not significant.
Median peak V’O2 for CVD patients was 54% lower than in control subjects. Exercise intolerance in patients with chronic (stable) heart failure is characterized by decreased maximal exercise capacity as well as slower adaptions to and from submaximal levels of exercise [29]. In a study of 18 men with chronic heart failure, using constant work rate cycle ergometry, Sietsema et al. [29] showed that peak V’O2 (corrected for weight) was 62% lower than in normal control subjects, similar to our findings. This difference remained essentially unchanged even when corrected for BIT (Figure 11). Furthermore, the O2-pulse (an index of stroke volume) in our patients with CVD was significantly less than in the COPD and control groups. Peak V’O2 and O2-pulse (an index of stroke volume) have strong predictive values for prognosis [30]. Reduced maximal exercise tolerance is associated with worse symptoms, diminished quality of life and survival, and a higher New York Heart Association functional class. Even if cardiac output is normal at rest, it usually does not increase enough with even mild exertion [31]. V’O2max is directly related to cardiac output and muscle perfusion at peak exercise [32,33]. Inability to raise cardiac output in response to increased metabolic demand leads to diminished perfusion to exercising muscles, including the diaphragm and other respiratory muscles, and contributes to dyspnea and muscle wasting [34]. Factors contributing to exercise intolerance particularly in patients with cardiomyopathy include diastolic as well as systolic dysfunction with impairment of stroke volume, down regulation of beta-receptors, pulmonary congestion and reduced lung compliance, increased pulmonary vascular resistance resulting in decreased cardiac output response to exercise [35] and limited metabolic capacity of peripheral skeletal muscles [36]. These factors undoubtedly contributed to the reduced exercise duration and encroachment on ventilatory reserve (Figure 6) in our CVD patients.
V’O2-BIT relationship
This relationship describes oxygen uptake for a given degree of ventilatory effort. It is analogous to the V’O2-work rate relationship used in cardiopulmonary exercise testing, and has the advantage of not having to use the esophageal balloon technique to directly measure the work produced by the respiratory muscles. While (V’O2)(BIT) was similar amongst the 3 cohorts at rest, a significant divergence in the products was noted at peak exercise, with control subjects exhibiting values 25% and 120% greater than the COPD and CVD cohorts, respectively. The change was almost entirely due to increases in the O2 uptake, as BIT did not differ amongst the 3 groups at peak exercise. CVD and COPD are both associated with impaired O2 transport [37]. In chronic heart failure, a blunted increase in cardiac output and stroke volume at peak exercise results in diminished blood flow and O2 supply to the respiratory muscles, resulting in decreased force generation to breathe with. The presence of interstitial edema reduces lung compliance, further increasing the work of breathing.
In the case of COPD, dynamic hyperinflation during exercise plays a major role in exercise intolerance and dyspnea. It might be expected that patients with the most severe airflow limitation would be the most dyspneic. Yet, some patients with severe airway obstruction, as reflected by the FEV1, have few symptoms, while others with minimal flow limitation exhibit severe dyspnea. In this connection, use of the negative expiratory pressure technique during quiet tidal breathing has shown that tEFL at rest is associated with lower inspiratory capacity (IC) [38]. Since maximal tidal volume correlates with IC during exercise, patients with tEFL at rest exhibit an increase in end-expiratory lung volume and a further decrease in IC with concomitant exercise limitation.
Relation of BIT to episodes of AECOPD and acute heart failure
Resting BIT was significantly higher in CVD patients who experienced 2 or more episodes of acute CHF; findings showed a similar trend in patients with lung disease experienced AECOPD, but their numbers did not reach significance. No patients required assisted ventilation despite their acute illness. Koga et al. [3] showed that patients with neuromuscular and chest wall disorders who required NIV exhibited significantly higher BIT values than those who did not. Since patients with patients with COPD and heart failure both exhibit respiratory muscle weakness and impaired function [5-8,20,22,39], one would expect similarly higher values of BIT in these patients as they developed respiratory insufficiency.
One might expect that BIT would reach higher values during AECOPD than in acute heart failure because respiratory muscle dysfunction in COPD develops as a result of mechanical disadvantage (hyperinflation) as well as progressive atrophy and impaired oxygen utilization. Dynamic hyperinflation and a reduced appositional area of the diaphragm (to the abdominal wall) are not features described in patients with acute heart failure, although the latter may exhibit both restrictive and obstructive defects [39,40] as well as respiratory muscle dysfunction related to decreased oxygen delivery [22].
Limitations of study
There were some limitations to this study. Conditions were identified on the basis of conventional clinical and diagnostic findings. The possibility that some patients with COPD harbored subclinical features of CVD, such as coronary artery disease or pulmonary hypertension, cannot be ruled out. Indeed, a recent meta-analysis has shown compelling evidence that COPD is associated with increased risk of common cardiovascular risk factors including smoking, diabetes and hypertension [41]. Patients with coexistent CVD or impaired oxygen-carrying capacity may exhibit impaired respiratory muscle oxygenation [8], therefore since the ability of these muscles to function anaerobically is limited [42], their ability to sustain spontaneous ventilation is ultimately limited by their aerobic capacity. Furthermore, increasing evidence suggests that cardiac dysfunction during AECOPDs is common and is associated with poor prognosis [43]. Lung volume measurements by body plethysmography were not available for all patients, so that we could not relate BIT or V’O2 to degree of lung hyperinflation.
Another limitation of this study is its retrospective design. However, patients were selected for analysis based on strict diagnostic criteria gleaned from medical records. Cohorts were maintained adherent to inclusion criteria as much as possible, assuring the homogeneity of the COPD and CVD groups. In addition, while there were only 20 subjects in each group, the strict inclusion criteria and high degree of significance of anthropometric and many physiologic variables allowed for discrimination amongst cohorts.
Conclusions
The BIT index is useful for evaluating respiratory muscle endurance under resting and exercise conditions in patients with CVD and COPD. Oxygen uptake fails to increase in proportion to BIT during cardiopulmonary exercise testing in these conditions as much as in healthy individuals, reflecting impaired oxygen transport and utilization. BIT may also be useful in determining those patients who are likely to develop acute decompensation from their illness, particularly by analyzing their breathing pattern at rest. Additional studies under resting and exercise conditions with larger cohorts would help determine if BIT predicts which patients are likely to develop AECOPD and acute heart failure, and perhaps the need for assisted ventilation.
Acknowledgements
The authors thank Dr. Joseph Milic-Emili (Emeritus Professor, McGill University, Montreal, QC, Canada) for helpful comments, the patients who underwent testing, and the technologists who performed the pulmonary function and cardiopulmonary exercise testing.
Conflict of Interest
The authors have no conflicts to declare.
References
- Bellemare F, Grassino A (1982) Effect of pressure and timing of contraction on human diaphragm fatigue.J ApplPhysiolRespir Environ ExercPhysiol 53: 1190-1195.
- Ramonatxo M, Boulard P, Préfaut C (1995) Validation of a noninvasive tension-time index of inspiratory muscles.J ApplPhysiol (1985) 78: 646-653.
- Koga T, Watanabe K, Sano M, Ishikawa Y, Bach JR (2006) Breathing intolerance index: a new indicator for ventilator use.Am J Phys Med Rehabil 85: 24-30.
- Baydur A, Chen Z (2013) Breathing intolerance index in COPD and obesity: A comparative observational study. Open J Respir Dis 3:119-127.
- Ambrosino N, Opasich C, Crotti P, Cobelli F, Tavazzi L, et al. (1994) Breathing pattern, ventilatory drive and respiratory muscle strength in patients with chronic heart failure.EurRespir J 7: 17-22.
- Hammond MD, Bauer KA, Sharp JT, Rocha RD (1990) Respiratory muscle strength in congestive heart failure.Chest 98: 1091-1094.
- McParland C, Krishnan B, Wang Y, Gallagher CG (1992) Inspiratory muscle weakness and dyspnea in chronic heart failure.Am Rev Respir Dis 146: 467-472.
- Mancini DM, Ferraro N, Nazzaro D, Chance B, Wilson JR (1991) Respiratory muscle deoxygenation during exercise in patients with heart failure demonstrated with near-infrared spectroscopy.J Am CollCardiol 18: 492-498.
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, et al. (2005) Interpretative strategies for lung function tests.EurRespir J 26: 948-968.
- Schoenberg JB, Beck GJ, Bouhuys A (1978) Growth and decay of pulmonary function in healthy blacks and whites.RespirPhysiol 33: 367-393.
- Crapo RO, Morris AH, Clayton PD, Nixon CR (1982) Lung volumes in healthy nonsmoking adults.Bull EurPhysiopatholRespir 18: 419-425.
- Milic-Emili J, Siafakas NM, Gautier H (1979) A new approach for clinical assessment of control of breathing.Bull EurPhysiopatholRespir 15 Suppl: 17-29.
- Parot S, Saunier C, Gautier H, Milic-Emili J, Sadoul P (1980) Breathing pattern and hypercapnia in patients with obstructive pulmonary disease.Am Rev Respir Dis 121: 985-991.
- Sorli J, Grassino A, Lorange G, Milic-Emili J (1978) Control of breathing in patients with chronic obstructive lung disease. Clin Sci Mol Med 54: 295-304.
- Aubier M, Murciano D, Fournier M, Milic-Emili J, Pariente R, et al. (1980) Central respiratory drive in acute respiratory failure of patients with chronic obstructive pulmonary disease.Am Rev Respir Dis 122: 191-199.
- Blom G (1958) Statistical estimates and transformed beta-variables. John Wiley & Sons, New York.
- Dixon WJ, Massey Jr FJ (1983) Introduction to statistical analysis, 4th edition, McGraw-Hill, New York, 385-414.
- McGill R, Tukey JW, Larsen W (1978) Variations of box plots. Amer Statistician 32:12-16.
- Bach JR, Goncalves M, Eisenberg M, Ishikawa Y, Altschuler E, et al. (2008) A ventilator requirement index.Am J Phys Med Rehabil 87: 285-291.
- Bellemare F, Grassino A (1983) Force reserve of the diaphragm in patients with chronic obstructive pulmonary disease.J Appl Physiol Respir Environ Exerc Physiol 55: 8-15.
- Al-Rawas OA, Carter R, Richens D, Stevenson RD, Naik SK, et al. (1995) Ventilatory and gas exchange abnormalities on exercise in chronic heart failure. Eur Respir J 8: 2022-2028.
- Vibarel N, Hayot M, Pellenc PM, Corret JL, Ramonatxo M, et al. (1998) Non-invasive assessment of inspiratory muscle performance during exercise in patients with chronic heart failure.Eur Heart J 19: 766-773.
- Tobin MJ, Chadha TS, Jenouri G, Birch SJ, Gazeroglu HB, et al. (1983) Breathing patterns. 1. Normal subjects.Chest 84: 202-205.
- García-Río F, Pino JM, Ruiz A, Díaz S, Prados C, et al. (2003) Accuracy of noninvasive estimates of respiratory muscle effort during spontaneous breathing in restrictive diseases.J Appl Physiol (1985) 95: 1542-1549.
- NHLBI Workshop summary (1990). Respiratory muscle fatigue. Report of the Respiratory Muscle Fatigue Workshop Group.Am Rev Respir Dis 142: 474-480.
- Vassilakopoulos T, Zakynthinos S, Roussos C (1998) The tension-time index and the frequency/tidal volume ratio are the major pathophysiologic determinants of weaning failure and success.Am J RespirCrit Care Med 158: 378-385.
- Elbehairy AF, Clavaglia CE, Webb KA,Guenette JA, Jensen D et al. (2015) Canadian Respiratory Research Network. Pulmonary gas exchange abnormalities in mild chronic obstructive pulmonary disease: implications for dyspnea and exercise intolerance. Am J RespirCrit Care Med. 191:1384-1394.
- Haouzi P, Young K (2015) Whether to breathe fast or not: What is wrong with breathing control in patients with mild obstructive pulmonary disease?Am J RespirCrit Care Med 192: 1524-1525.
- Sietsema KE, Ben-Dov I, Zhang YY, Sullivan C, Wasserman K (1994) Dynamics of oxygen uptake for submaximal exercise and recovery in patients with chronic heart failure.Chest 105: 1693-1700.
- Balady GJ, Arena R, Sietsema K, Myers J, Coke L, et al. (2010) American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; and Interdisciplinary Council on Quality of Care and Outcomes Research. Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 122:191-225.
- Reddy HK, Weber KT, Janicki JS, McElroy PA (1988) Hemodynamic, ventilatory and metabolic effects of light isometric exercise in patients with chronic heart failure.J Am CollCardiol 12: 353-358.
- Stringer WW, Hansen JE, Wasserman K (1997) Cardiac output estimated noninvasively from oxygen uptake during exercise.J ApplPhysiol (1985) 82: 908-912.
- Stork M, Novak J, Zeman V (2011) Cardiac output estimation based on oxygen consumption during exercise test on bicycle ergometer. MEASUREMENT 2011. Proceedings of the 8th International Conference, Smolenice, Slovakia, 289-292.
- Harrington D, Anker SD, Chua TP, Webb-Peploe KM, Ponikowski PP, et al. (1997) Skeletal muscle function and its relation to exercise tolerance in chronic heart failure.J Am CollCardiol 30: 1758-1764.
- Butler J, Chomsky DB, Wilson JR (1999) Pulmonary hypertension and exercise intolerance in patients with heart failure.J Am CollCardiol 34: 1802-1806.
- Piña IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, et al. (2003) Exercise and heart failure: A statement from the American Heart Association Committee on exercise, rehabilitation, and prevention.Circulation 107: 1210-1225.
- Sun XG, Hansen JE, Oudiz RJ, Wasserman K (2002) Gas exchange detection of exercise-induced right-to-left shunt in patients with primary pulmonary hypertension. Circulation 105:54-60.
- Koulouris NG, Dimopoulou I, Valta P, Finkelstein R, Cosio MG, et al. (1997) Detection of expiratory flow limitation during exercise in COPD patients.J Appl Physiol (1985) 82: 723-731.
- Dimopoulou I, Daganou M, Tsintzas OK, Tzelepis GE (1998) Effects of severity of long-standing congestive heart failure on pulmonary function.Respir Med 92: 1321-1325.
- Light RW, George RB (1983) Serial pulmonary function in patients with acute heart failure.Arch Intern Med 143: 429-433.
- Chen W, Thomas J, Sadatsafavi M, FitzGerald JM (2015) Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis.Lancet Respir Med 3: 631-639.
- Manohar M, Hassan AS (1990) Diaphragm does not produce ammonia or lactate during high-intensity short-term exercise.Am J Physiol 259: H1185-1189.
- MacDonald MI, Shafuddin E, King PT, Chang CL, Bardin PG, et al. (2016) Cardiac dysfunction during exacerbations of chronic obstructive pulmonary disease.Lancet Respir Med 4: 138-148.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences