Acute Effects of Inspiratory Muscle Training on Pulmonary Arterial Pressure,Cardiac Output and Pulmonary Arterial Saturation Derived Oxygen Cost of Breathing
Sven Stieglitz, Sandhya Matthes, Christina Priegnitz, Lars Hagmeyer, Winfried Randerath
DOI10.21767/2572-5548.100023
Sven Stieglitz1, Sandhya Matthes2, Christina Priegnitz3, Lars Hagmeyer3 and Winfried Randerath3
1Petrus Hospital Wuppertal, Academic Teaching Hospital of the University of Duesseldorf, Department of Pneumology and Cardiology, Carnaper Str. 48, 42283 Wuppertal, Germany
2LMU Hospital of the University of Munich, Medical Department V, Germany
3Clinic for Pneumology and Allergology, Centre for Sleep and Ventilation Medicine Bethanien Hospital, Institute of Pneumology, University Witten, Germany
- Corresponding Author:
- Stieglitz S
Petrus Hospital Wuppertal
Academic Teaching Hospital of the University of Duesseldorf
Department of Pneumology and Cardiology
Carnaper Str. 48, 42283 Wuppertal, Germany
Tel: 0049-151-18-266 995
Fax: 0049-202-299-2507
E-mail: sven.stieglitz@cellitinnen.de
Received Date: December 16, 2016; Accepted Date: January 12, 2017; Published Date: January 19, 2017
Citation: Stieglitz S, Matthes S, Priegnitz C, Hagmeyer L, Randerath W (2017) Acute Effects of Inspiratory Muscle Training on Pulmonary Arterial Pressure, Cardiac Output and Pulmonary Arterial Saturation Derived Oxygen Cost of Breathing. Chron Obstruct Pulmon Dis 2:23. doi 10.21767/2572-5548.100023
Copyright: © 2017 Stieglitz S et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Respiration and respiratory maneuvers affect cardiac output. Inspiratory muscle training (IMT) increases the negative thoracic pressure, which could have an impact on intra-thoracic hemodynamics and thereby influence the cardiac output (CO). The objective of our study was to determine changes in pulmonary arterial (PA) hemodynamics and the CO during IMT in patients with suspected pulmonary hypertension. A new method for measuring the oxygen cost of breathing during IMT was also developed. 22 patients were included in this prospective study. They performed IMT during right heart catheterization. Mixed-venous blood gas analysis was performed before and at the end of IMT to calculate the oxygen cost of breathing. The baseline PA pressure was systolic/diastolic/mean (s/d/m) 41 ± 20/13 ± 20/25 ± 11 mmHg. The CO was 5.3 ± 1.5 l/min. The IMT was set to 2.3 ± 0.6 kPa (23.7 ± 5.7 cmH2O). The PA pressure at the end of IMT was s/d/m 44/10/22 mmHg s/d/m. Though there was a trend towards a lower diastolic pressure, this change was not statistically significant (p=0.06). CO (6.2 ± 1.1 l/min) did not change due to IMT. The mixed-venous hemoglobin oxygen saturation was 71.8 ± 2.3% before IMT, with a significant reduction to 65.8 ± 5.9% (p=0.027) at the end of IMT. IMT as performed in our study neither changed the mean PA pressure nor the CO. Measuring the oxygen cost of breathing is feasible by the method described in our paper.
Keywords
Cardiorespiratory interaction; COPD; Heart failure; Oxygen cost of breathing; Ventilation
Introduction
Respiration and respiratory maneuvers affect cardiac output. During inspiration usually right ventricular stroke volume is increased while the left ventricular stroke volume is decreased. In recent years, inspiratory muscle training (IMT) programs have been developed, mainly in COPD [1,2] though inspiratory muscle dysfunction can also be observed in non-pulmonary diseases like chronic heart (failure) CHF [3]. It could be demonstrated, that in COPD, IMT improves the strength of inspiratory muscles, endurance, functional capacity, dyspnea and quality of life [4]. In CHF, IMT improves dyspnea as well as the general performance [5-7].
The basic principle of the most common IMT consists of breathing in for some minutes through a device that impedes inspiration by a threshold (threshold loading). The use of an inspiratory threshold valve has been also suggested during cardiopulmonary resuscitation because this results in a greater negative intra-thoracic pressure which enhances the return of the blood to the thorax [8]. Therefore, we hypothesized that an IMT also leads to an increase of CO. This is an important question not only in patients with CHF but also in COPD as cardiac comorbidities are common (in 64% of the patients an abnormal echocardiography may be found [9]). Furthermore, both COPD and CHF may also lead to pulmonary hypertension (PH), which is defined by a resting mean pulmonary artery pressure (mPAP) of ≥25 mmHg [10]. Current guidelines recommend using a pulmonary arterial wedge pressure to distinguish between pre-capillary (≤15 mmHg) and post-capillary (>15 mmHg) PH, the latter indicating left heart disease [11]. The prevalence of PH in patients listed for lung transplantation with severe COPD is about 30% [12]. Results of other studies show a prevalence of up to 50% [13]. In fact, in more than 90% of patients with COPD a mean pulmonary arterial pressure (mPAP) above 20 mmHg may be observed [14].
Epidemiological data (prevalence of PH in left heart disease reported in studies is between 25-100%) of the exact incidence of PH in patients with cardiac disease are not available [15]. Nevertheless, PH is common not only in patients with reduced ejection fraction (EF) but also in those with elevated left ventricular filling pressures, which can also lead to PH independently of the ejection fraction [16,17]. Furthermore remodeling may lead to an additional pre-capillary component in PH due to left heart disease [18,19]. Notably, it was shown that the inspiratory muscles in PAH are also impaired and that maximal inspiratory mouth pressure (Pimax) is reduced by a third, [20] which has been shown in some studies to correlate with the 6MWD (in contrast, hemodynamic characteristics assessed during right heart catheterization were not correlated with 6MWD) [21].
Until now, the acute hemodynamic effects of IMT irrespectively of the presence of PH have not been investigated. The objective of our study was to determine an increase of CO and therefore also of the PAP during inspiratory muscle training in patients with suspected PH and to measure the oxygen cost of breathing due to IMT.
Materials and Methods
The study was performed in agreement with the declaration of Helsinki. The research protocol was approved by the local Ethics Committee (University of Witten-Herdecke, Witten- Herdecke, Germany) and all patients gave their written informed consent.
Study design
We conducted a prospective study from January 2009 to December 2013. Patients who required right heart catheterization (RHC) for clinical reasons (e.g., clarification of dyspnea, pulmonary hypertension or right heart failure) were enrolled in the study. All patients were clinically stable. The Threshold-IMT (Philips Respironics, Amsterdam, the Netherlands) was used for IMT. Before the RHC was performed, patients were taught how to use the Threshold-IMT by respiratory therapists and practiced IMT under the supervision of therapists. Each unit of training consisted of 7 repetitions with 2 min IMT followed by 1 min of rest. During IMT, inspiration is carried out via a mouthpiece with a variable threshold that may be changed from 7 to 41 cmH2O. Inclusion criteria were indication for RHC and age >18 years. Exclusion criteria were lack of written consent and inability to perform IMT. Lung function parameters were measured according to actual guidelines via body plethysmography (Masterlab, Carefusion, San Diego, USA) [22]. The load imposed on the inspiratory muscles was assessed by calculating the P0.1 (mouth occlusion pressure after 0.1 s of inspiration). Pimax (maximal inspiratory mouth pressure) and P0.1/Pimax (respiratory capacity) were measured according to ATS/ERS recommendations [23]. RHC was performed in a supine position and blood sampling (central venous, right atrium, right ventricle) were performed as recommended in a review [24]. Pulmonary arterial wedge pressure was performed as recommended recently [10]. Cardiac output was calculated by thermodilution (5 mL; bolus 0 degree of Celsius, average of three bolus). All hemodynamic parameters were measured before IMT and during IMT (exactly 2 min after initiation of the IMT).
After measurement of the hemodynamic parameters (by Ultraview SL, Spacelabs, Snoqualmie, Washington, USA) and assessment of the blood samples, patients were switched to a sitting position. After recalibration, patients performed IMT for at least 2 minutes. The Threshold-IMT incorporates a flowindependent one-way valve to ensure a constant resistance. The specific pressure was set as follows: 20-30% of Pimax but depending on individual tolerance a higher resistance was attempted. If Pimax was very low, the lowest possible pressure was set at the Threshold-IMT. During IMT (exactly 2 min after initiation of the IMT) PAP and CO were measured and a blood gas sample from the PA was obtained. Blood gas samples were analyzed by ABL 800, Radiometer, Copenhagen Denmark.
Analysis
The primary endpoint and null hypothesis of the study was an increase of the mPAP in a mixed patient population. Sample size calculation was based on the assumption of a clinical relevant increase of mPAP at the end of an IMT with a difference of 5 mmHg in mean. With a standard deviation of 9 mmHg, α=0.05, and one side testing, the sample size of 22 was calculated for dependent testing to be sufficient to guarantee a power of 80% in this study. The Wilcoxon test was used to calculate the hemodynamic changes. Secondary endpoints were changes of cardiac output and stroke volume. In addition we measured the oxygen cost of breathing in some of the patients. SPSS software (version 20; IBM, Armonk, New York, USA) was used for statistical analysis. Results are presented by with an interquartile range of the 25th and 75th percentile or means ± standard deviations (as indicated). Subgroup analysis was performed in patients with only COPD or only chronic heart failure.
Results
22 patients (10 men, 12 women) were investigated. The Pimax was (mean ± standard deviation) 6.2 ± 2.8 kPa (63.1 ± 29.0 cmH2O), Pimax % pred 59.5 ± 27.6%, P0.1 0.3 ± 0.2 kPa (3.3 ± 2.2 cmH2O) and the P0.1% 142.3 ± 98.3. For complete anthropometric and lung function data (Table 1).
| n | 22 |
|---|---|
| Women/men (n) | 10-Dec |
| Age (years) | 67.4 ± 12.4 |
| BMI (kg/m2) | 28.2 ± 5.5 |
| COPD (n) | 4 |
| Left heart failure (n) | 10 |
| Interstitial lung disease (n) | 1 |
| Left heart failure and interstitial lung disease (n) | 3 |
| COPD and left heart failure (n) | 4 |
| ITGV (litres) | 3.9 ± 1.3 |
| ITGV (% pred) | 121.5 ± 59.6 |
| FEV1 (litres/sec) | 1.8 ± 0.8 |
| FEV1 (% pred) | 72.4 ± 29.5 |
| VC (litres) | 2.6 ± 0.9 |
| VC (% pred) | 79.6 ± 25.8 |
| R (kPA) | 0.5 ± 0.4 |
| R (% pred) | 150.0 ± 128.6 |
| Pimax (kPA) (cmH2O) | 6.2 ± 2.8 (63.1± 29.0) |
| Pimax(% pred) | 59.5 ± 27.6% |
| P0.1 (kPA) (cmH2O) | 1 0.3 ± 0.2 (3.3 ± 2.2) |
| P0.1 (% pred) | 142.3 ± 98.3 |
| PO2 (mmHg) | 60.7 ± 8.5 |
| PCO2 (mmHg) | 38.5 ± 6.7 |
Table 1: Anthropometric characteristics and lung function parameters. ITGV=intrathoracic gas volume. FEV1=forced exspiratory volume in 1 second. VC=vital capacity. R=resistance. Pimax=maximal inspiratory pressure. P0.1=mouth pressure at 0.1 seconds. Capillary blood gas analysis from the ear lobe (PO2, PCO2). Values are expressed as means ± SD.
The median of the baseline PAP was systolic/diastolic/mean (s/d/m) 36/13/22 (25th-75th percentile 25-54/5 -19/13–35) mmHg (mean mPAP 25 ± 14 mmHg). The pulmonary arterial wedge pressure (PAWP) was 10 (4-14) mmHg. The cardiac output was 5.2 l/min (4.4–6.5), cardiac index CI 2.7 l/min/m2 (2.4-3.2) and stroke volume index SVI 39.5 [mL/min/m2] (28.6– 59.7). The pulmonary vascular resistance (PVR) was calculated 145 (107-349) [dynes cm-5] (Table 2).
| n | 22 |
|---|---|
| CVP (mmHg) | 4 (1-8) |
| RA (mmHg) | 4 (1-8) |
| PA s/d/m (mmHg) | 36/13/22 (24.5-53.5/5.0 -18.5/12.8-34.5) |
| PAWP (mmHg) | 10 (4-14) |
| CO (litres/min) | 5.2 (4.4-6.5) |
| CI (litres/min/m2) | 2.7 (2.4-3.2 |
| Heart rate (/min) | 78 (70-99) |
| SVI (mL/min/m2) | 39.5 (28.6-59.7) |
| PVR (dynes·s·cm-5) | 145 (107-349) |
| SVR (dynes·s·cm-5) | 1352 (904-1671) |
| SPAO2 (%) | 71.8 ± 2.3 |
Table 2: Hemodynamic baseline parameters. CVP=central venous pressure. RA=pressure right atrium. SPAO2=Hemoglobinsaturation of the pulmonary artery. Data are expressed as median with interquartile range (25th–75th percentile except SPAO2 (mean ± SD).
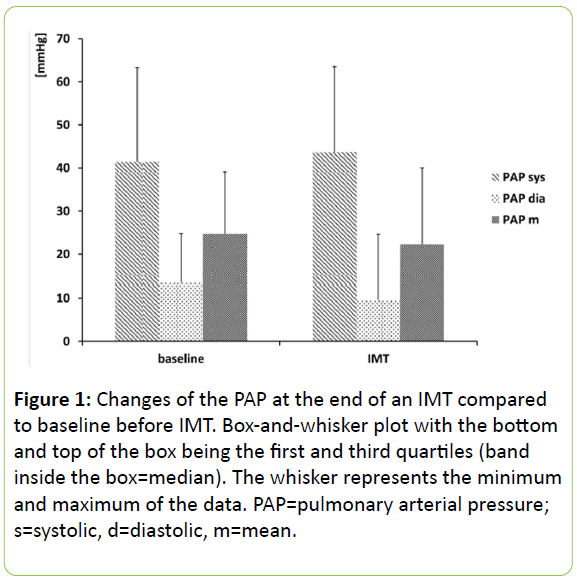
The IMT was set to 2.3 ± 0.6 kPa (23.7 ± 5.7 cmH2O). IMT was performed for 2.2 ± 0.3 min. The s/d/m PAP at the end of IMT was 41/9/19 mmHg (26-59/-1-22/9-37). Though there was a trend towards a lower diastolic pressure, there was no statistically significant change (p=0.06) (Figure 1).
Figure 1: HPLC chromatogram of the nine reference compounds in 50% aqueous methanol, measured at 370nm. Retention times for rutin, sutherlandin A, sutherlandin B, kaempferol-3-O-rutinoside, sutherlandin C, sutherlandin D, quercitrin, quercetin and kaempferol were 11.9, 12.7, 13.8, 15.3, 16.2, 17.0, 18.0, 26.2 and 28.1 minutes, respectively.
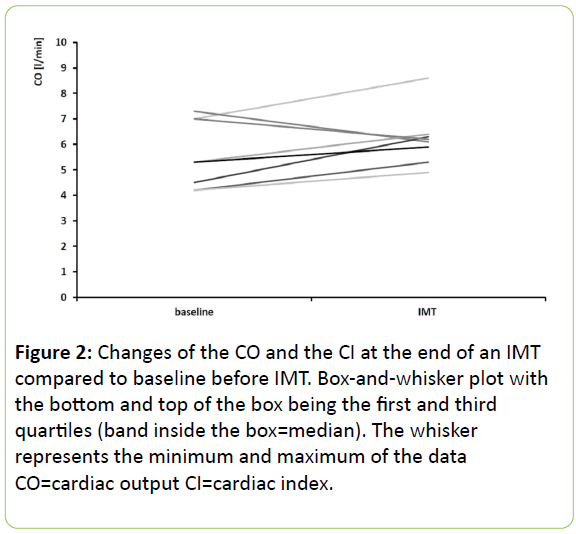
At the end of IMT cardiac output (6.1; 5.5-6.4 l/min) and cardiac index (3.0; 2.9-3.3 l/min/m²) were measured in 8 patients which did not change compared to baseline (CO p=0.20 and CI p=0.18) (Figure 2).
Figure 2: HPLC chromatogram of the nine reference compounds in 50% aqueous methanol, measured at 370nm. Retention times for rutin, sutherlandin A, sutherlandin B, kaempferol-3-O-rutinoside, sutherlandin C, sutherlandin D, quercitrin, quercetin and kaempferol were 11.9, 12.7, 13.8, 15.3, 16.2, 17.0, 18.0, 26.2 and 28.1 minutes, respectively.
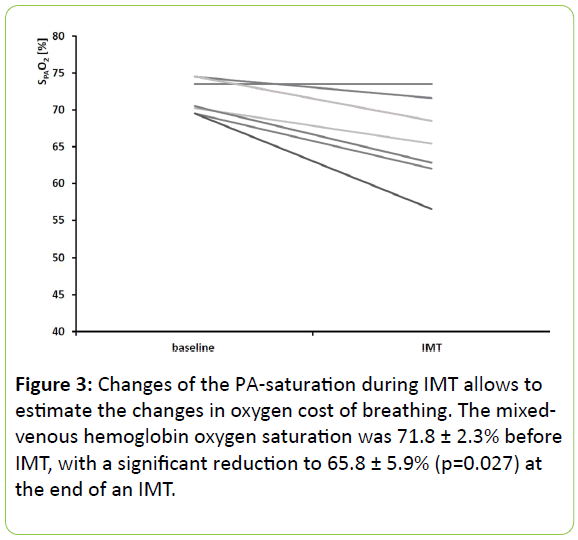
In 7 patients mixed-venous hemoglobin oxygen saturation was obtained at the end of IMT.
The mixed-venous hemoglobin oxygen saturation of these patients was 71.8 ± 2.3% (mean ± SD) before IMT and showed a significant reduction to 65.8 ± 5.9% (p=0.027) at the end of IMT (Figure 3).
Figure 3: HPLC chromatogram of the nine reference compounds in 50% aqueous methanol, measured at 370nm. Retention times for rutin, sutherlandin A, sutherlandin B, kaempferol-3-O-rutinoside, sutherlandin C, sutherlandin D, quercitrin, quercetin and kaempferol were 11.9, 12.7, 13.8, 15.3, 16.2, 17.0, 18.0, 26.2 and 28.1 minutes, respectively.
Subgroup analysis of patients with only COPD or heart failure did not lead to any other significant findings.
Discussion
In our study, the systolic and the mean PA pressure during IMT for 2.2 min with 23.7 cmH2O (2.3 kPa) did not change. We would classify the intensity of the IMT as moderate. The median mPAP in the study population was not elevated above ≥ 25 mmHg, so a pulmonary arterial hypertension was excluded.
Spontaneously breathing patients usually have a fall in the pleural pressure during inspiration. One of the factors determining whether this leads to a decrease in the right atrial pressure [25] is the fluid load. During IMT, this effect should be aggravated. The mean PAP is also linked to the CO and an exercise-induced pulmonary hypertension may occur. Nevertheless, in our study we did not observe any change of CO that might explain at least in part why the mPAP did not change at the end of the IMT trial.
Increasing intra-thoracic pressure by coughing or the Valsava manoeuvre leads to an increase in the pulmonary arterial pressure [26]. During IMT, inspiration against a threshold-a contrary manoeuver-is conducted and therefore a reduction of the intra-thoracic pressure should be expected as well as a decrease in pulmonary arterial hypertension. Indeed, in our study there is a trend towards a lower diastolic PA pressure (p=0.06). One may suppose that the result is not statistically significant due to the sample size or the low intensity of the performed IMT.
CO after 2 minutes of an IMT could only be measured in 8 patients, because the patients often stopped IMT before three bolus could be performed. We believe that it was extremely important to measure the CO only during the IMT and that measuring after stopping the IMT would cause a bias. We did not find any change of the CO at the end of IMT. This is surprising because as the process of IMT may be regarded as an increased inspiration one could expect an increase in venous return to the right ventricle with a subsequent increase in the pulmonary venous pressure. Inspiratory threshold valves may be used in resuscitation where in fact an increase in cardiac output a blood pressure may be achieved [27].
On the other side, an increase in the negative pressure during inspiration may cause an increase in left ventricular afterload by increasing the transmural pressure of the left ventricle which consequently results in a fall in the left ventricular output [28]. In this case, inspiratory threshold loading would be expected to cause a reduction in cardiac output which may in turn have deleterious effects on inspiratory muscle function with an increase in the oxygen cost of breathing and deoxygenation of the respiratory muscle [29]. We also know that upper airway obstruction (e.g., by tracheal tumor or tonsillar hypertrophy) may cause pulmonary edema [30] due to fluid shift induced by the drop of the negative intra-thoracic pressure generated in the thorax when the inspiration is carried out against a closed glottis or obstructed upper airway.
In another scenario concerning patients with COPD and intrinsic positive-endexpiratory pressure (iPEEP) of the lung, the following sequence following IMT is imaginable: during inspiration prior to iPEEP being overcome, pressure around the right ventricle decreases whilst alveolar pressure does not change, so the right ventricle must generate higher pressures, i.e., right ventricular afterload increases in order to counterbalance negative transmural pressure, [31] which could lead to a potential leftward shift of the septum that causes a decrease in LV dimension and filling (SV reduced).
In our study, we describe for the first time a new method how to measure the oxygen consumption of the inspiratory muscles. Oxygen cost of breathing can be measured by subtracting the oxygen consumption at rest from that observed during an increased respiratory activity [32]. Most commonly it is obtained from the slope of the oxygen uptake and ventilation relationship [33-35]. In our study we employed an entirely different method: changes of the oxygen consumption of the inspiratory muscles may also be obtained by sampling blood during right heart catheterization (mixed-venous blood). The oxygen cost of breathing during IMT may be estimated then by measuring the difference of the pulmonary oxygen saturation before and at the end of a period of IMT. This method is described for the 1st time (in terms of proof of concept). It was only possible to obtain the blood gas sample of a minority of patients because we intended to obtain the blood sample exactly two minutes after the start of an on-going period of IMT. Most of the patients stopped the IMT before blood sampling was possible. In cases where a measurement was possible, we found that the PA saturation was reduced by 6% due to IMT. The extent of this change was surprising for us because we believe that the intensity of the IMT was modest. There is another reason why these findings are remarkable: in earlier studies a high negative inspiratory pressure increased the diffusion capacity for carbon dioxide by 6% due to recruitment of the pulmonary capillary bed which in turn should rather increase the saturation [36]. Further studies should evaluate the potential of pulmonary arterial saturation derived oxygen cost of breathing as a monitoring tool for respiratory muscles in intensive care (e.g., during spontaneous breathing trials).
In quiet breathing, the oxygen cost of breathing is extremely small, being less than 5% of the resting oxygen consumption. However with voluntary hyperventilation, it is possible to increase it up to 30%. In patients with COPD, the oxygen cost of breathing may limit their exercise ability [37]. In CHF, a maladaptive breathing pattern (increase in inspiratory and decrease in expiratory time) [28] and structural changes of the diaphragm with fast to slow heavy chains with a decrease in glycolytic capacity [38] develop. Taken all together, diaphragmatic work and therefore oxygen cost of breathing is dramatically increased in patients with HF and approaches levels shown to generate fatigue. This contributes to the sensation of dyspnea which is closely related to respiratory muscle function [39]. As demonstrated by a recent study, [40] cardiac and pulmonary function may be improved by exercise training.
Limitations
First of all, IMT was performed only with a single adjustment. Because we did not perform IMT without threshold it is impossible to estimate the influence of deep inspiration on the oxygen cost of breathing. Secondly, due to methodological reasons assessment of CO and oxygen cost of breathing was not obtained in all patients. Thirdly, the standard deviation of our results is high, so that the conclusions have to be drawn carefully. And finally, the patients in our study had a moderate PH and it remains unclear if our findings are reproducible in patients with a normal PA pressure.
Conclusion
Performing IMT in patients with moderate PH has no impact on the PA pressure and cardiac output is also not affected. Moderately performed IMT increases the oxygen cost of breathing by 6%.
Acknowledgement
Grateful thanks to Thomas Hilberg from the University of Wuppertal, Institute for Sport Science and Sports Medicine, for his enthusiasm and support.
References
- Decramer M (1985) Response of the respiratory muscles to rehabilitation in COPD. J ApplPhysiol 107:971-976.
- Held HE, Pendergast DR (2014) The effects of respiratory muscle training on respiratory mechanics and energy cost. RespirPhysiolNeurobiol200:7-17.
- Meyer FJ, Borst MM, Zugck C, Kirschke A, Schellberg D, et al. (2001) Respiratory muscle dysfunction in congestive heart failure: clinical correlation and prognostic significance. Circulation 103:2153-2158.
- Gosselink R, De Vos J, van den Heuvel SP, Segers J, Decramer M, et al. (2011) Impact of inspiratory muscle training in patients with COPD: what is the evidence? EurRespir J 37:416-425.
- Adamopoulos S, Schmid JP, Dendale P, Poerschke D, Hansen D, et al. (2014) Combined aerobic/inspiratory muscle training vs. aerobic training in patients with chronic heart failure: The Vent-HeFT trial: a European prospective multicentre randomized trial. Eur J Heart Fail.
- Sutbeyaz ST, Koseoglu F, Inan L, Coskun O (2010) Respiratory muscle training improves cardiopulmonary function and exercise tolerance in subjects with subacute stroke: a randomized controlled trial. ClinRehabil 24:240-250.
- Darnley GM, Gray AC, McClure SJ, Neary P, Petrie M, et al. (1999) Effects of resistive breathing on exercise capacity and diaphragm function in patients with ischaemic heart disease. Eur J Heart Fail 1:297-300.
- Lurie K, Voelckel W, Plaisance P, Zielinski T, McKnite S, et al. (2000) Use of an inspiratory impedance threshold valve during cardiopulmonary resuscitation: a progress report. Resuscitation 44:219-230.
- Freixa X, Portillo K, Pare C, Garcia-Aymerich J, Gomez FP, et al. (2013) Echocardiographic abnormalities in patients with COPD at their first hospital admission. EurRespir J 41:784-791.
- Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, et al. (2013) Definitions and diagnosis of pulmonary hypertension. J Am CollCardiol 62:D42-50.
- Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, et al. (2016) ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37:67-119.
- Cuttica MJ, Kalhan R, Shlobin OA, Ahmad S, Gladwin M, et al. (2010) Categorization and impact of pulmonary hypertension in patients with advanced COPD. Respir Med. 104:1877-1882.
- Thabut G, Dauriat G, Stern JB, Logeart D, Levy A, et al. (2005) Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest 127:1531-1536.
- Scharf SM, Iqbal M, Keller C, Criner G, Lee S, et al. (2002) Hemodynamic characterization of patients with severe emphysema. Am J RespirCrit Care Med166:314-322.
- Vachiery JL, Adir Y, Barbera JA, Champion H, Coghlan JG, et al. (2013) Pulmonary hypertension due to left heart diseases. J Am CollCardiol62:D100-8.
- Aragam JR, Folland ED, Lapsley D, Sharma S, Khuri SF, et al. (1992) Cause and impact of pulmonary hypertension in isolated aortic stenosis on operative mortality for aortic valve replacement in men. Am J Cardiol 69:1365-1367.
- Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, et al. (2009) Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am CollCardiol 53:1119-1126.
- Moraes DL, Colucci WS, Givertz MM (2000) Secondary pulmonary hypertension in chronic heart failure: the role of the endothelium in pathophysiology and management. Circulation 102:1718-1723.
- Delgado JF, Conde E, Sanchez V, Lopez-Rios F, Gomez-Sanchez MA, et al. (2005) Pulmonary vascular remodeling in pulmonary hypertension due to chronic heart failure. Eur J Heart Fail 7:1011-1016.
- Meyer FJ, Lossnitzer D, Kristen AV, Schoene AM, Kubler W, et al. (2005) Respiratory muscle dysfunction in idiopathic pulmonary arterial hypertension. EurRespir J 25:125-130.
- Kabitz HJ, Schwoerer A, Bremer HC, Sonntag F, Walterspacher S, et al. (2008) Impairment of respiratory muscle function in pulmonary hypertension. ClinSci (Lond)114:165-171.
- Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, et al. (2005) Standardisation of the measurement of lung volumes. EurRespir J 26:511-522.
- American Thoracic Society/European Respiratory S (2002) ATS/ERS Statement on respiratory muscle testing. Am J RespirCrit Care Med 166:518-624.
- Rosenkranz S, Behr J, Ewert R, Ghofrani HA, Grunig E, et al. (2011) [Right heart catheterization in pulmonary hypertension]. Dtsch Med Wochenschr 136:2601-2616.
- Michard F, Teboul JL (2000) Using heart-lung interactions to assess fluid responsiveness during mechanical ventilation. Crit Care 4:282-289.
- Opotowsky AR, Ojeda J, Rogers F, Arkles J, Liu T, et al. (2010) Blood pressure response to the valsalva maneuver. A simple bedside test to determine the hemodynamic basis of pulmonary hypertension. J Am CollCardiol 56:1352-1353.
- Lurie KG, Zielinski T, McKnite S, Aufderheide T, Voelckel W (2002) Use of an inspiratory impedance valve improves neurologically intact survival in a porcine model of ventricular fibrillation. Circulation 105:124-129.
- Hart N, Kearney MT, Pride NB, Green M, Lofaso F, et al. (2004) Inspiratory muscle load and capacity in chronic heart failure. Thorax 59:477-482.
- Mancini DM, Ferraro N,Nazzaro D, Chance B, Wilson JR (1991) Respiratory muscle deoxygenation during exercise in patients with heart failure demonstrated with near-infrared spectroscopy. J Am CollCardiol18:492-498.
- Fremont RD,Kallet RH, Matthay MA, Ware LB (2007)Postobstructive pulmonary edema: a case for hydrostatic mechanisms. Chest 131:1742-1746.
- Ranieri VM, Dambrosio M, Brienza N (1996) Intrinsic PEEP and cardiopulmonary interaction in patients with COPD and acute ventilatory failure. EurRespir J 9:1283-1292.
- McGregor M, Becklake MR (1961) The relationship of oxygen cost of breathing to respiratory mechanical work and respiratory force. J Clin Invest 40:971-980.
- Lorenzo S, Babb TG (2012) Oxygen cost of breathing and breathlessness during exercise in nonobese women and men. Med Sci Sports Exerc 44:1043-1048.
- Katsardis CV, Desmond KJ, Coates AL (1986) Measuring the oxygen cost of breathing in normal adults and patients with cystic fibrosis. RespirPhysiol 65:257-266.
- Field S, Kelly SM, Macklem PT (1982) The oxygen cost of breathing in patients with cardiorespiratory disease. Am Rev Respir Dis 126:9-13.
- Cotton DJ, Mink JT, Graham BL (1983) Effect of high negative inspiratory pressure on single breath CO diffusing capacity. RespirPhysiol54:19-29.
- >West JB, West JB (1998) Pulmonary pathophysiology--the essentials. Baltimore, Md.: Williams & Wilkins (5th edn) p: 198.
- Tikunov B, Levine S, Mancini D (1997) Chronic congestive heart failure elicits adaptations of endurance exercise in diaphragmatic muscle. Circulation 95:910-916.
- Mancini DM, Henson D, LaManca J, Levine S (1992) Respiratory muscle function and dyspnea in patients with chronic congestive heart failure. Circulation 86:909-918.
- Acanfora D, Scicchitano P, Casucci G, Lanzillo B, Capuano N, et al. (2016) Exercise training effects on elderly and middle-age patients with chronic heart failure after acute decompensation: A randomized, controlled trial. Int J Cardiol225:313-323.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences