Thrombomodulin for Acute Exacerbations of Idiopathic Pulmonary Fibrosis
Yasunori Ichimura, Kenji Tsushima, Takuma Matsumura, Mitsuhiro Abe, Koichiro Tatsumi
DOI10.21767/2572-5548.100024
Yasunori Ichimura1, Kenji Tsushima2,3*, Takuma Matsumura3, Mitsuhiro Abe3 and Koichiro Tatsumi3
1St. Luke's International University, Graduate School of Public Health, Japan
2Department of Pulmonary Medicine, International University of Health and Welfare, Japan
3Department of Respirology, Graduate School of Medicine, Chiba University, Japan
- *Corresponding Author:
- Kenji Tsushima
Department of Pulmonary Medicine
International University of Health and Welfare, Japan
Tel: +81 287-24-3000
E-mail: tsushimakenji@yahoo.co.jp
Received date: June 08, 2017; Accepted date: June 19, 2017; Publidhed date: June 26, 2017
Citation: Ichimura Y, Tsushima K, Matsumura T, Abe M, Tatsumi K (2017) Thrombomodulin for Acute Exacerbations of Idiopathic Pulmonary Fibrosis. Chron Obstruct Pulmon Dis Vol No 2 Iss No 1:24. doi: 10.21767/2572-5548.100024
Copyright: © 2017 Ichimura Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Acute exacerbation (AE) is known as a leading cause of morbidity and mortality in chronic fibrosing group includes idiopathic pulmonary fibrosis (IPF) and nonspecific interstitial pneumonia (NSIP). There is no standardizes drug therapy with comparative studies. The clinical application of recombinant human soluble thrombomodulin (rhTM) has recently been expected against AE-IPF/NSIP, however, the impact of rhTM remains unestablished. Thus, this review assessed the impact of rhTM on mortality in patients with AE-IPF/NSIP.
Methods: PubMED database was investigated for studies in English on May 2017 by two independent reviewers. The investigated period was restricted to previous 10 years. Studies was included adults with AE of IPF or idiopathic interstitial pneumonias, and treatment with thrombomodulin. Key data on study design, patient characteristics, outcome measures and adverse events were extracted for the review. We pooled mortality using random-effects meta-analysis
Results: Three prospective and two retrospective studies were included. The five studies recruited a total of 77 patients receiving rhTM treatment (rhTM group). Corticosteroid therapy was examined in all studies for both rhTM groups and non-rhTM group. Mortality at 28 days or one month was evaluated in all three prospective studies, and was significantly lower in the rhTM group than in nonrhTM group. The pooled 1-month mortality was significantly lower in rhTM group (Odds Ratio (OR): 0.25 (95% CI, 0.08-0.77)). Mortality at 3 months was also lower in rhTM group. In patients with AE-IPF/NSIP, rhTM administration was of significant prognostic value of 3-month mortality by univariate analysis and by multivariate analysis. Two retrospective studies reported adverse effect in rhTM group and/or non-rhTM group.
Conclusion: In this study, a decline in mortality was confirmed when rhTM was administered in treating AE-IPF/ NSIP, in conjunction with steroid therapy and immunosuppressive drugs. It indicated a possibility of rhTM administration being effective against AE-IPF/NSIP.
Keywords
Pulmonary Fibrosis; Idiopathic Interstitial pneumonias; Acute exacerbation; Pneumonia
Background
The idiopathic interstitial pneumonias (IIPs) are diffuse parenchymal lung diseases with unknown etiology. The updated American Thoracic Society (ATS) and European Respiratory Society (ERS) IIPs Classification 2013 distinguished major IIPs from rare IIPs and unclassifiable cases [1]. Major IIPs are grouped by clinical disease behavior, and chronic fibrosing group includes idiopathic pulmonary fibrosis (IPF) and nonspecific interstitial pneumonia (NSIP). Most of them follow progressive irreversible course.
Acute exacerbation is a clinical condition of rapidly progressing respiratory failure accompanied by new developments of infiltrative shadows in both lungs observed during the chronic course of progressive interstitial pneumonia [2]. Acute exacerbation is known to develop during IPF clinical course, and, in 2011 evidence-based IPF guidelines, it is clearly stated as “acute exacerbation” [3]. In 2016, “An International Working Group Report” on acute exacerbation has been published [4]. In this report, new definitions and diagnostic criteria of acute exacerbation were proposed. Also in this report, subcategories of “triggered acute exacerbation” and “idiopathic acute exacerbation” were proposed. The number of reports of acute exacerbation in other chronic progressive interstitial pneumonias besides IPF is also increasing. The frequency of the acute exacerbation in IPF varies in reports, but it is reported to be around 5-15% annually [5].
Acute exacerbation is one of the decisive factors of prognoses of interstitial pneumonia itself. The mortality of the acute exacerbation of IPF (AE-IPF) is considered to be 70-80%, and it is a clinical condition with extremely adverse prognosis [5]. There has been no established drug therapy for acute exacerbation so far that has been proven by comparative studies. In the actual clinical treatments, steroid therapies such as pulse therapy are sometimes conducted. Developments of effective drug therapies for this pathological condition are much anticipated.
Recombinant human soluble thrombomodulin (rhTM) not only exerts antithrombin action by bonding to thrombin but also has anticoagulant activity by acting on thrombomodulin-Protein C (PC)-PC receptor system. It is expected to be developed as a new remedy for disseminated intravascular coagulation (DIC) [6]. Japanese Clinical Practice Guidelines for Sepsis 2016 didn’t announce clear recommendation on rhTM pharmaceutical preparations toward septic DIC patients [7]. However, the systematic review having carried out in these guidelines stated that the effect estimation value of therapeutic intervention of rhTM in septic DIC was Relative Risk (RR): 0.81 (95% confidence interval (CI), 0.62-1.06), whose result was that the benefit probably would exceed the damage [7]. In recent years, rhTM has been known to have not only anticoagulant action but also anti-inflammatory action [8]. In AE-IPF, neutrophils accumulated in the lung injures vascular endothelium, which causes inflammation, congealing fibrinogenolysis system abnormality and fibrillation. The clinical application of rhTM is thus expected against AE-IPF and a few prospective and retrospective studies have been reported gradually in recent years.
The objective of this paper was to summarize and evaluate the available evidence of the use of rhTM for acute exacerbations in patients with chronic progressive fibrosing interstitial pneumonias such as IPF and to provide suggestion for its role in the treatment of acute exacerbation.
Methods
We conducted a systematic literature search for study publications using National Institutes of Health PubMed database. The key search terms were “pulmonary fibrosis” AND thrombomodulin, or “interstitial pneumonia” AND thrombomodulin, by two independent reviewers. We didn’t include the word of “acute exacerbation” in PubMed search, on the grounds that the terminology related to AE-IPF or idiopathic interstitial pneumonias differs among reports. The searched period was restricted to previous 10 years. The searches were performed on May 2017, for studies in English.
The results from the search were assessed based on titles and abstracts. Studies had to include adults with AE-IPF or idiopathic interstitial pneumonias, and treatment with thrombomodulin. After that, those relevant publications were obtained and evaluated in the review. An overlapped study was excluded from the review.
Key data on study design, patient characteristics (eg, age, sex, and the oxidation capability), outcome measures and adverse events were extracted for the review. We pooled mortality using random-effects meta-analysis
Results
Search and selection results
A total of 11 citations were identified, and after screening, five publications were included in the review [9-13]. All studies were reported from Japan.
Study characteristics
All enrolled studies are listed in Table 1. Of enrolled five studies, three were prospective, historical control studies [9,10,13] and two were retrospective studies (11,12). The five studies recruited a total of 77 patients receiving rhTM treatment (rhTM group).
| Study | Study design | Year | Objectives | Size | Dose of rhTM | Other Treatment |
|---|---|---|---|---|---|---|
| Tsushima 2014 | prospective historical control | 2010-2012 | AE-IPF | rhTM group:20 non-rhTM group:6 |
0.06 mg/kg/day for 6 days | *CS pulse therapy for 3 days, followed by maintenance treatment with a tapered dose of CS *NIPPV or mechanical ventilation (if necessary) |
| Abe 2015 | prospective historical control | 2012-2014 | AE-IPF/NSIP | rhTM group: 11 non-rhTM group: 11 |
0.06 mg/kg/day for 6 days | *CS pulse therapy for 3 days, followed by maintenance treatment with a tapered dose of CS *cyclophosmide and/or cyclosporine *NIPPV |
| Hayakawa 2016 | prospective historical control | 2012-2014 | AE-IPF | rhTM group: 10 non-rhTM group: 13 |
380 U/kg/day for 7 days | *CS pulse therapy for 3 days, followed by maintenance treatment with a tapered dose of CS *mechanical ventilation (if necessary) |
| Isshiki 2015 | retrospective | 2006-2013 | AE-IPF | rhTM group: 16 non-rhTM group: 25 |
0.06 mg/kg/day for 6 days | *CS pulse therapy for 3 days, followed by maintenance treatment with a tapered dose of CS *cyclosporine (for almost all patients) *LMWH (for some patients in control group) *NIPPV or mechanical ventilation (if necessary) |
| Kataoka 2015 | retrospective historical control | 2009-2011 | AE-IPF | rhTM group: 20 non-rhTM group: 20 |
0.06 mg/kg/day for 6 days (followied by LMWH administration) |
*CS pulse therapy for 3 days, followed by maintenance treatment with a tapered dose of CS *cyclosporine *LMWH (for non-rhTM group) *NIPPV or mechanical ventilation (if necessary) |
| AE: Acute Exacerbation; IPF: Idiopathic Pulmonary Fibrosis; NSIP: Non-Specific Interstitial Pneumonia; rhTM: Recombinant Human Soluble Thrombomodulin; LMWH: Low-Molecular-Weight Heparin; CS: Corticosteroid; NPPV: Noninvasive Positive-Pressure Ventilation. | ||||||
Table 1: The characteristics of five reviewed studies.
Patient characteristics
The mean age of rhTM group in the studies was similar (ranged from 69 to 76 years old), and the number of male patients included in total were approximately sextuple the number of female patients (130:22). Patients in rhTM group had lower SOFA score (3.3,7.3) and/or APACHE II score (9.0-12), but there was no significant difference in these scores between rhTM group and patients treated without rhTM (non-rhTM group). Patients in rhTM group had also lower PaO2/FIO2 (PF) ratio, meanwhile the range of PF ratio was relative wide (107-258) among studies. In rhTM group, higher coagulation markers such as thrombin–antithrombin complex and D-dimer were noted in all studies.
Treatment Regimen
For rhTM group, the administration of rhTM was started at a dose of 0.06 mg/kg/day once daily for six days, in four of five studies [10-13]. The treatment with rhTM was followed by continuous intravenous infusion of low-molecular weight heparin (LMWH) (750,000 IU/kg/day) in one study [11]. Remaining one prospective study adopted a different dose and duration of rhTM, as at a dose of 380 IU/kg/day once a day for seven days [9]. Corticosteroid therapy was examined in all studies for both rhTM groups and non-rhTM group.
Mortality
All studies evaluated mortality, but the difference of mortality between the rhTM and non-rhTM group was seen in the periods after beginning treatment with rhTM.
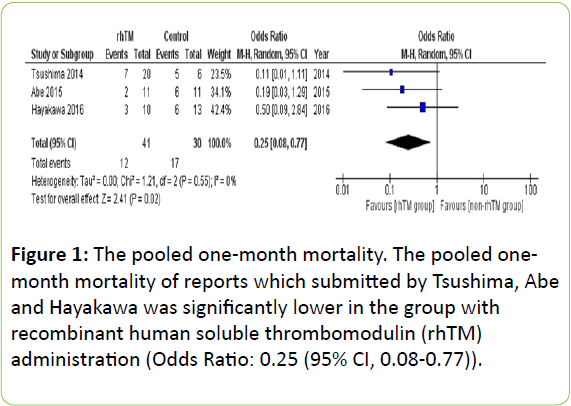
Mortality at 28 days or one month was evaluated in all three prospective studies, and was lower in the rhTM group than in non-rhTM group (7/20 (35%) vs 5/6 (83%), 2/11 (18%) vs 6/11 (54%) and 3/10 (30%) vs 6/13 (46%), respectively) [9,10,13]. As shown in Figure 1, the pooled 1-month mortality was significantly lower in rhTM group (Odds Ratio (OR): 0.25 (95% CI, 0.08-0.77)).
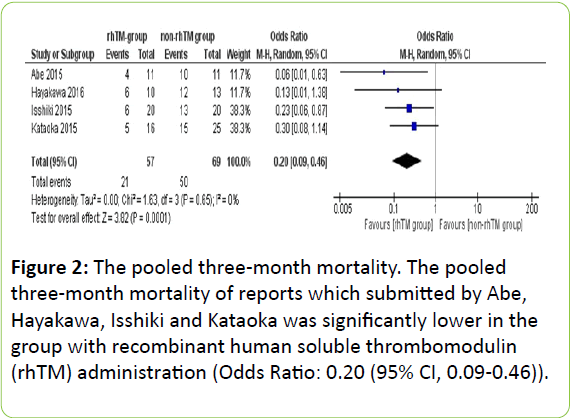
Mortality at 3 months was evaluated in four of five studies [9-12]. It was lower in the rhTM group than in non-rhTM group in all studies. As shown in Figure 2, the pooled 3-month mortality was also significantly lower in rhTM group in two prospective studies (OR: 0.08 (95% CI, 0.02-0.46)) [9,10], and in two retrospective studies (OR: 0.26 (95% CI, 0.10-0.68)) [11,12].
Univariate and multivariate analysis of predictors of 3-month mortality
In patients with AE-IPF/NSIP, rhTM administration was of significant prognostic value of 3-month mortality by univariate analysis in three of three studies [10-12] and by multivariate analysis in two of two studies [10,11]. Higher lactate dehydrogenase (LDH) was of significant prognostic value by univariate analysis in one study [12]. Higher C reactive protein (CRP) was of prognostic significance in another study by univariate analysis [11].
Adverse effect
Two retrospective studies reported adverse effect in rhTM group and/or non-rhTM group [11,12]. There was one patient with mild hemoptysis and hematuria in rhTM group, but no patient with severe bleeding in one study [12]. Another study reported one patient with hemoptysis and one patient with acute deep vein thrombosis in rhTM group [11]. This study also reported two patients with bleeding in non-rhTM group, who received LMWH administration. There was no adverse event attributable to rhTM in two prospective studies [9,10].
Discussion
Three prospective and two retrospective studies were included. The five studies recruited a total of 77 patients receiving rhTM treatment. Corticosteroid therapy was examined in all studies for both rhTM and non-rhTM groups. Mortality at 28 days or one month was evaluated in all three prospective studies, and was lower in the rhTM group than in non-rhTM group. The pooled 1-month mortality was significantly lower in rhTM group (OR: 0.25 (95% CI, 0.08-0.77)). Mortality at three months was also lower in rhTM group, and the pooled 3-month mortality was also significantly lower in rhTM group in two prospective studies (OR: 0.08 (95% CI, 0.02-0.46)), and in two retrospective studies (OR: 0.26 (95% CI, 0.10-0.68)). In patients with AE-IPF/NSIP, rhTM administration was of significant prognostic value of 3-month mortality by univariate analysis and by multivariate analysis. Two retrospective studies reported adverse effect in rhTM group and/or non-rhTM group, but there was no adverse event attributable to rhTM in two prospective studies.
In this analysis, pretreatment coagulation abnormality but not DIC was observed in all studies. In the two studies that followed the time course of coagulation abnormality, it was observed that the abnormality improved after rhTM treatment. It has been suggested that, in terms of the pathological physiology of AE-IPF, various factors such as alveolar epithelial injury, inflammation, cytokine and matrix metalloproteinase are involved, and coagulation abnormality is one of them [14]. There have been several papers reporting on acute exacerbation and coagulation abnormality as well [14,15]. The clinical trial that evaluated the effectiveness of warfarin against IPF did not prove effectiveness of the administration of warfarin, but instead, the warfarinadministered group showed increased fatality rate comparing with the placebo group, and the trial was suspended [16]. However, in study conducted in Japan, warfarin and LWMH showed favorable effects against IPF [17]. Administration of anticoagulant drug against IPF is currently evaluated negatively, but as the association of coagulation abnormality with AE-IPF has been reported as mentioned above, the necessity of controlling coagulation function is indicated against such cases [18].
In Japan, rhTM is widely used as anticoagulant drug against DIC. A systematic review and a meta-analysis were conducted on septic DIC [6]. In this study, although the relative risk of fatality in RCT did not show significant decreasing trend (RR, 0.81; 95% CI, 0.62-1.06), the observation study observed its significant decrease (RR, 0.59; 95% CI, 0.45-0.77). The meta recurrence analysis in each study design observed a significant negative correlation between the effective amount of rhTM treatment and the baseline mortality (p=0.012).
The use of rhTM has contributed to anti-inflammatory effect of thrombomodulin in addition to that of active protein which has been indicated since before [8]. Therefore, rhTM may be superior than anticoagulant such as warfarin to AE-IPF in which inflammation is associated with clinical condition. Tsushima mentioned the anti-inflammatory action by rhTM contributed to the efficacy in AE-IPF [13]. It has been reported about its side effect that no significant difference was observed in severe hemorrhagic complication between rhTM group and control group [6]. Even though hemorrhage in small number of cases in retrospective studies was reported in the current consideration [11,12], no severe hemorrhage was recognized. In addition, any adverse event was not reported in prospective studies [9,10]. Therefore, administration of rhTM is believed to be a therapy with tolerability.
Limitations
First, all the study cases we selected into this study happened to be reports from Japan. The possibility of Japanese being more subject to AE-IPF compared to other races has been pointed out [19]. The possibility of genetic factor for AE-IPF is also speculated. Secondly, in the study cases included in this analysis, steroid therapy, and in many cases, immunosuppressive drugs were used. In such combination therapies, no difference was observed between rhTM group and non-rhTM group (in one study case, immunosuppressive drugs were administered in only non-rhTM group [9]), and this could have influenced the outcomes. Thirdly, all the study cases of this study were smallscale clinical studies. Also, the prospective studies were conducted as historical control, and the rest are all retrospective studies. Currently, the phase III trial is being conducted in collaboration of multiple facilities, and its outcomes need to be compared to the results of this analysis. Fourthly, although the mortality selected as outcome measures was clinically important, there were differences in the period of mortality after the start of rhTM treatment, among studies. The analysis observed effectiveness of rhTM administration at both onemonth and three-month periods. However, it may require setting a common endpoint in the future.
Conclusion
In this study, a decline in mortality was confirmed when rhTM was administered in treating AE-IPF/NSIP, in conjunction with steroid therapy and immunosuppressive drugs. This was confirmed through five small-scale studies, which indicated a possibility of rhTM administration being effective against AE-IPF/ NSIP. Since the selected studies were small-scale prospective/ retrospective studies, the effectiveness of rhTM must be further examined through comparing the results from the randomized control trial being conducted at present and the results from this study.
References
- Travis WD, Costabel U, Hansell DM, King TE, Lynch DA, et al. (2013) An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J RespirCrit Care Med188:733-748.
- Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, et al. (2007) Acute exacerbations of idiopathic pulmonary fibrosis. Am J RespirCrit Care Med 176:636-643.
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, et al. (2011) An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J RespirCrit Care Med183:788-824.
- Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, et al. (2016) Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J RespirCrit Care Med194:265-275.
- Society TJR (2016) Idiopathic Interstitial Pneumonias: Diagnosis and Treatment (3rd Edn) Japan.
- Yamakawa K, Aihara M, Ogura H, Yuhara H, Hamasaki T(2015) Recombinant human soluble thrombomodulin in severe sepsis: a systematic review and meta-analysis. J ThrombHaemost13:508-519.
- The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2016 ÃÆïÃâüÃâÃâ J-SSCG2016ÃÆïÃâüÃâââ¬Â°The diagnosis adn treatment of DIC in sepsis (2017). J Japanese Association Acute Medi28:174-186.
- Abeyama K, Stern DM, Ito Y, Kawahara K, Yoshimoto Y, Tanaka M, et al. (2005) The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest115:1267-1274.
- Hayakawa S, Matsuzawa Y, Irie T, Rikitake H, Okada N, et al. (2016) Efficacy of recombinant human soluble thrombomodulin for the treatment of acute exacerbation of idiopathic pulmonary fibrosis: A single arm, non-randomized prospective clinical trial. MultidiscipRespir Med11:38.
- Abe M, Tsushima K, Matsumura T, Ishiwata T, Ichimura Y, et al. (2015) Efficacy of thrombomodulin for acute exacerbation of idiopathic pulmonary fibrosis and nonspecific interstitial pneumonia: a nonrandomized prospective study. Drug Des DevelTher 9:5755-5762.
- Kataoka K, Taniguchi H, Kondoh Y, Nishiyama O, Kimura T, et al. (2015) Recombinant Human Thrombomodulin in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Chest 148:436-443.
- Isshiki T, Sakamoto S, Kinoshita A, Sugino K, Kurosaki A, et al. (2015) Recombinant human soluble thrombomodulin treatment for acute exacerbation of idiopathic pulmonary fibrosis: a retrospective study. Respiration89:201-217.
- Tsushima K, Yamaguchi K, Kono Y, Yokoyama T, Kubo K, et al. (2014)Thrombomodulin for acute exacerbations of idiopathic pulmonary fibrosis: a proof of concept study. PulmPharmacolTher29:233-240.
- Collard HR, Calfee CS, Wolters PJ, Song JW, Hong SB, et al. (2010) Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell MolPhysiol299:3-7.
- Ware LB, Matthay MA, Parsons PE, Thompson BT, Januzzi JL, et al. (2007)Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med35:1821-1828.
- Noth I, Anstrom KJ, Calvert SB, de Andrade J, Flaherty KR, et al. (2012) A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am J RespirCrit Care Med 186:88-95.
- Kubo H, Nakayama K, Yanai M, Suzuki T, Yamaya M, et al. (2005) Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest128:1475-1482.
- Juarez MM, Chan AL, Norris AG, Morrissey BM, Albertson TE (2015) Acute exacerbation of idiopathic pulmonary fibrosis-a review of current and novel pharmacotherapies. J Thorac Dis7:499-519.
- Azuma A, Hagiwara K, Kudoh S (2008) Basis of acute exacerbation of idiopathic pulmonary fibrosis in Japanese patients. Am J RespirCrit Care Med 177:1397-1398.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences